Interferons (IFNs) are proteins made and released by host cells in response to the presence of pathogens or tumor cells. Being able to "interfere" with viral replication within host cells,Interferon blocks virus replication by inducing the expression of interferon-stimulated genes (ISGs) which were reported to block viral replication and regulate immune responses. However, the underlying molecular mechanisms of these ISGs are largely unknown, including IFN-induced tetratricopeptide repeats (IFITs), also known as IFN-stimulated gene 56 (ISG56).
 |
|
|
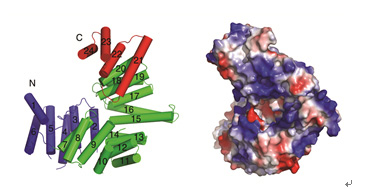
Figure1 The crystal structure of IFIT5/ISG58. The crystal structure shows that IFIT5 consists of 24 α-helices that pack into a special “V”-like structure (left panel). Electrostatic surface representation of IFIT5 is shown on right panel. | |
Recently, Dr. LIANG Huanhuan and co-authors from LIU Yingfang’s group at the Institute of Biophysics, Chinese Academy of Sciences (IBP) made important progress in this field by collaborating with Prof. ZENG Su’s group at Zhejiang University and Prof. CHENG Genhong’s group at UCLA.Professor LIU is a leading structural biologist in China who studies of proteins related to cancer, virus, replication and apoptosis. Previously, using structural biology methods, LIU’s group found that IFIT2/ISG54 possessed a right-handed helical channel which recognizes AU-rich RNAs. They also proved that the RNA binding ability of IFIT2 was related to its antiviral activity (Cell Research. 2012).
Other researchers have also shown that IFIT1/ISG56 specifically recognizes viral 5’-pppRNA. As the expression patterns of IFITs have been reported to be cell- and inducer-specific, LIU’s group hypothesizes that different family members likely recognize distinct substrates.
In a recent study on IFIT5/ISG58, Dr. LIANG and her colleagues found that different from other IFITs which are oligomers in solution, ISG58 is a stable monomer. It folds into “V” shape and has a smaller nucleotide binding channel at its C-terminus. Compared with IFIT1 and IFIT2 which bind to 5’-pppRNA and AU-rich RNA, respectively, IFIT5 binds to both ss-RNA and ds-DNA in vitro. They concluded that the differences in the C-terminal structures of IFITs may provide a structural basis for the differences in their nucleotide binding patterns.
Dr. LIANG Huanhuan and her colleagues published these results in the latest issue of Cell Research, as a continuation of their previous study on IFIT2. When this paper was under revision, the structure of IFIT5 was almost simultaneously reported by two other groups who published their results in Nature and Molecular Cell. These results show that ISG58 binds to certain types of RNA, and that its RNA-binding ability is important to its antiviral activity.
Although further study of the exact function of the nucleotides binding activity is still needed, results from this study suggest that the nucleotides binding ability may enable IFIT5 to recognize both RNA and DNA viruses, thus expanding the virus-recognition spectrum of IFITs.
This work was a fruit of cooperation among scientists from the Institute of Biophysics, Chinese Academy of Sciences, Zhejiang University, China and University of California, USA
link:http://www.nature.com/cr/journal/vaop/ncurrent/full/cr201380a.html