G Protein Coupled Receptor (GPCR), which contributes to the development of modern pharmacology, is one of the most important drug targets. Ten Nobel Prizes have been awarded to the research related to the field of GPCR, including the latest one in 2012. In GPCR research, there are two general signaling pathways mediating the majority of GPCR functions, namely, G protein and Arrestin. However, the detailed mechanisms of how G protein or Arrestin recognizes specific GPCR information and translates into signaling output remain unclear.
In a new study published on Sep. 8 in Nature Communications, entitled Phospho-selective Mechanisms of Arrestin Conformations and Functions Revealed By Unnatural Amino Acid Incorporation and 19F-NMR, Prof. WANG Jiangyun’s group from Institute of Biophysics, together with Prof. SUN Jinpeng from Shangdong Univesity, discover that Arrestin dictates signaling-mediated mechanisms by means of recognizing GPCR specific phosphor-coding information and put forward important new hypothesis.
For decades, receptor C-terminal phosphorylation barcodes (phosphorylation by different kinases) have been hypothesised to dictate distinct receptor signalling through arrestins. This hypothesis may also explain the diverse functions of various GPCRs in different cellular contexts. However, the exact phospho-barcode message and the accompanying mechanism for this signalling control had not been defined. For example, there is no defined phosphorylation sequence information that correlates with their distinct Arrestin-mediated functions. In addition, the conformational heterogeneity of Arrestin poses significant challenges for these studies. As a consequence, certain researchers even doubt about the existence of the true “phospho-barcode” of the receptor, callingit a “floating hypothesis”.
Therefore, the mechanisms of receptor phosphorylation barcode and a structural understanding of the phosphorylation coded specific Arrestin conformations are in great need to clarify these uncertainties. Thus, Prof. WANG Jiangyun and Prof. SUN Jinpeng used novel unnatural amino acid incorporation technology and 19F-NMR spectroscopy to address this question. The application of site-directed 19F-NMR enabled us to decipher the specific receptor-phospho-code read by Arrestins. For example, whereas a phospho-binding sequence of 1-4-6-7 pattern determines the specific β-arrestin-1 conformation for clathrin recruitment, a different receptor ‘tune,’ 1-5, provides the SRC signaling order. Moreover, further 19F-NMR and cellular studies demonstrated that distinct Arrestin conformational states induced by specific phosphorylation patterns are coupled to their selective functions, which are applied to many GPCR members. Based on these discoveries, these studies brought up a “flute model” for GPCR-Arrestin working mechanism: The receptor phospho-C-tail works like the fingers of a magic hand, manipulating music from the receptor by touching on the N-terminal hole of the Arrestin (like a flute). The combination of the fingers generates different signalling tunes.
In general, the combination of all phosphate binding sites in single Arrestin member accommodates more than 1,000 patterns (210 – 1= 1023) that can produce a plethora of Arrestin conformations for numerous downstream protein interactions. Therefore, phospho-binding concave rendered enough space for its recognition of plethora phosphorylation states of numerous GPCRs,which generate specific Arrestin conformations for selective functions, to shape one side for functional diversity of many GPCRs (more than 800 in human genome).
This study has laid a solid foundation for us to understand the important mechanisms of GPCR phosphor coding.
Full Text
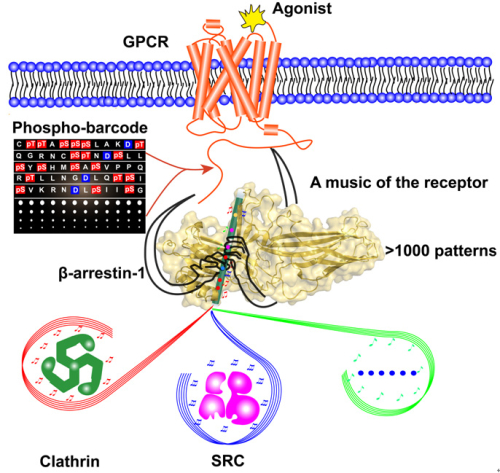
Fig: Arrestin mediated signaling directed by phospho-coding of the receptor (image by WANG Jiangyun’s group)
Contact:
WANG Jiangyun
Institute of Biophysics, Chinese Academy of Sciences
Website: http://english.ibp.cas.cn/ibp_pi/W/201312/t20131212_114365.html
Email: jwang@ibp.ac.cn