As a serious global health problem that affects approximately 170 million people worldwide, the hepatitis C virus (HCV) infection frequently lead to cirrhosis and hepatocellular carcinoma. The transcription factor nuclear factor κB(NF-κB) is crucial for innate immune defense against viral infections, and its activation requires the ubiquitylation of upstream proteins, including the adaptor protein NEMO (NF-κB essential modulator).
Many infectious pathogens, including HCV, inhibit NF-κB signaling in host cells, which promotes pathogen survival. A high proportion of HCV-infected individuals develop chronic infections, suggesting that HCV can subvert host antiviral responses. However, the detailed mechanisms remain to be elucidated.
In a new study published in Science Signaling, Prof. DENG Hongyu’s group, from Institute of Biophysics, CAS, released their findings on how HCV subverts host antiviral responses.
Innate immunity is the first defending line against viral infections. Normally, the inflammatory cytokine tumor necrosis factor-α (TNF-α) induces antiviral innate immune responses by stimulating NF-κB through a cascade of signaling events. However, patients, who are chronically infected with HCV, have increased serum amounts of TNF-α, but have ineffective immune responses due to the inhibited NF-κB activation.
The researchers hypothesized that the inhibition of TNF-α–induced NF-κB activation might be mediated by a viral protein expressed during HCV infection. Thus, they used a luciferase reporter assay to screen which viral protein suppresses TNF-α–induced NF-κB activation. They found that NS3 was sufficient to block NF-κB signaling in cells. Therefore, they hypothesized that NS3 might inhibit TNF-α–induced NF-κB activation through its interaction with linear ubiquitin chain assembly complex (LUBAC). The coimmunoprecipitation and immunofluorescence staining experiments showed that the HCV protein NS3 directly interacted with LUBAC. Domain mapping experiments showed NS3 is bound to the ZnF-RBZ domain of HOIP (HOIL-1L–interacting protein; also known as RNF31), which is the same domain in LUBAC that binds to NEMO. Thus, they hypothesized that the binding of NS3 to HOIP might interfere with the interaction between HOIP and NEMO. To test that, researchers performed competitive coimmunoprecipitation experiments. They found that overexpression NS3 indeed disrupted the interaction between HOIP and NEMO in a concentration-dependent manner, and the HCV protein NS3 directly interacted with LUBAC, competed with NEMO for binding to LUBAC, and inhibited the LUBAC-mediated linear ubiquitylation of NEMO as well as the subsequent activation of NF-κB.
Their results highlight a novel immune evasion strategy adopted by HCV to modulate host antiviral responses and enhance virus survival and persistence.
This study was supported by National Natural Science Foundation and the Ministry of Science and Technology (973 Program) of China.
Full Text
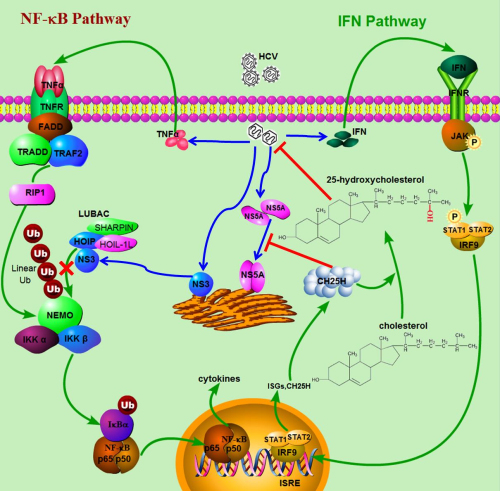
Figure: Interactions between HCV and the host innate immune system(Image by IBP, CAS).
Contact:
DENG Hongyu
Institute of Biophysics, Chinese Academy of Sciences
http://english.ibp.cas.cn/ibp_pi/AG/201311/t20131128_113437.html
Email: hydeng@moon.ibp.ac.cn
CHENG Genhong
UCLA