Using cryo-electron microscopy, an international group of scientists have reported a potent HAV-specific neutralizing monoclonal antibody R10 that blocks receptor attachment and interferes viral uncoating. High resolution cryo-EM structures of HAV full and empty particles and of the complex of HAV with R10 Fab reveal the atomic details of antibody binding and point to a receptor recognition site at the pentamer interface.
HAV is unique amongst picornaviruses in targeting the liver, and despite a successful vaccine continues to be a source of mortality. It has radically different properties from other picornaviruses, for instance it exists in an enveloped form and is unusually stable, both genetically and physically. In 2015, IBP scientists solved crystal structure of unenveloped HAV showing no trace of the canyon which encircles the 5-fold axes of enteroviruses and is often the site of receptor binding. However, the molecular basis where the cellular receptor TIM-1 might attach remains unknown.
Professor RAO Zihe and Professor WANG Xiangxi at the Institute of Biophysics (IBP) of the Chinese Academy of Sciences, in cooperation with Professor David Stuart in Oxford University and colleagues applied cryo-electron microscopy to determine high resolution cryo-EM structures of HAV full particles, empty particles and full particles complexed with R10 Fab, revealing that R10 binds to the viral surface along the edges of the pentameric building block of the virus, and these interactions are critical for receptor binding and viral uncoating.
In their studies, a fluorescent assay revealed that while the R10 Fab portion destabilizes full HAV particles by 7 °C. Given the ability of the R10 Fab to destabilize HAV, the binding sites on HAV of the host factor and R10 may overlap partially or fully. To explore the mechanism of neutralization, the researchers performed the real-time reverse transcriptase-PCR (RT-PCR) assay to quantify the virus remaining on the cell surface, following exposure to antibodies pre- and post-virus attachment to cells at 4 °C. They found R10 prevents HAV attachment to the cell surface and is able to displace virus that has already bound to the cell receptor.
The research work entitled Potent neutralization of Hepatitis A virus reveals a receptor mimic mechanism and the receptor recognition site was published on line in the journal PNAS on January 10, 2017. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, the Ministry of Science and Technology 973 Project and National Science Foundation.
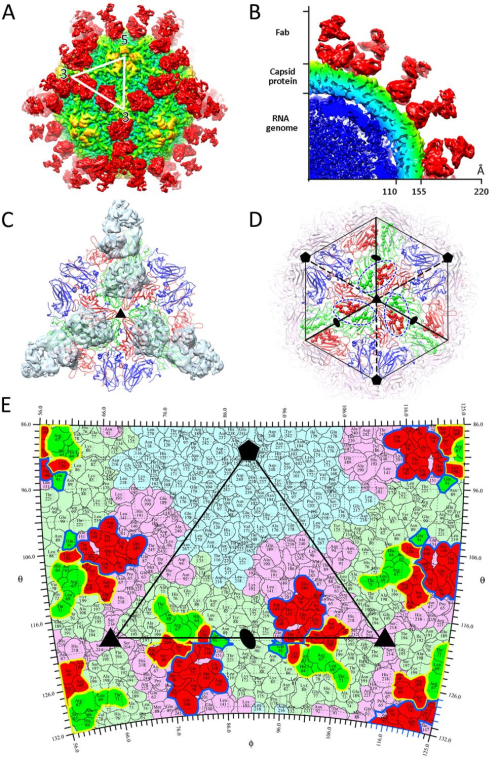
Fig.1 Overall structure of HAV virus complexed with R10 Fab (Image by IBP)
Contact
RAO Zihe
National Laboratory of Biomacromolecules, IBP
E-mail:raozh@sun5.ibp.ac.cn
Tel:010-64888556