TDP-43 is a multifunctional DNA- and RNA- binding protein involved in various cellular processes, including RNA transcription, alternative splicing and mRNA stability regulation. Since the landmark discovery of TDP-43 as a major component of the inclusion bodies in ALS (amyotrophic lateral sclerosis) and FTLD (frontotemporal lobar degeneration), more than 30 mutations have been identified in individuals affected by ALS and FTLD. However, molecular mechanisms by which TDP-43 mutations lead to neurotoxicity remain to be elucidated.
Professor WU Ying and her colleagues at the Institute of Biophysics, Chinese Academy of Sciences had developed a Drosophila model for TDP-43 proteinopathy (PNAS, 2010). They showed that expression of human TDP-43 in a variety of neurons recapitulates the features of neurodegenerative disease. Recently, Guo et al. in WU’s lab further demonstrated that expression of the wildtype (Wt) and an ALS-associated mutant, A315T, of TDP-43 in fly motoneurons causes axonal and cell body swelling, as well as decreased mobility and viability (Figure 1). The C-terminal region of TDP-43, which flanks residue 315, shows sequence similarity to prion proteins, and is predicted to form β-sheet structure. Synthetic peptides of the Wt and the A315T mutant of TDP-43 form amyloid fibrils in vitro (Figure 2), and cause neurotoxicity in primary neuronal cultures, with mutant peptide exhibiting enhanced neurotoxicity. Their work provides evidence for the structural and biochemical similarities between TDP-43 and prion proteins, and suggests that TDP-43 derivatives may cause spreading of the disease phenotype among neighboring neurons.
These new findings were published online in a research article titled “An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity” by Nature Structural & Molecular Biology on June 12th 2011. This work was completed in collaboration with Professors SHEN Yan and XU Qi from PUMC and Professor WANG Chen from NCNST, and funded by the Ministry of Science and Technology of China 973 Project and the Chinese Academy of Sciences.
(http://www.nature.com/nsmb/journal/v18/n7/full/nsmb.2053.html)
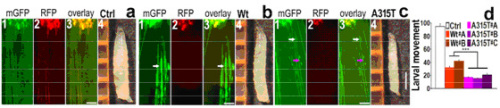
Figure 1. Expression of A315T mutant hTDP-43 in fly motor neurons (MNs) leads to enhanced axonal damage and more severe impairment of locomotive function. by WU Ying et al.
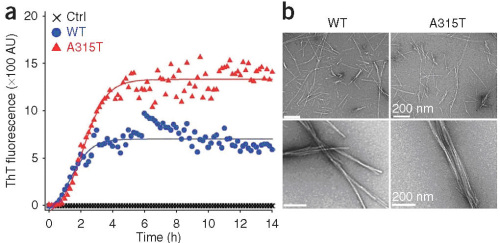
Figure 2. Synthetic TDP-43 peptides (WT or A315T mutant) form fibrils in vitro. by WU Ying et al.
by CHEN Yanbo