The process of protein synthesis in vivo is a highly complex and orderly system, which is dependent on the ribosome as factory, mRNA as a template, the amino acid as raw materials, the GTP for energy. A large number of outstanding scientists work to resolve the structure of the ribosome in a series of functional status. Although People have in-depth understanding about the choice of the aminoacyl-tRNA on the ribosome, the molecular mechanism of peptide bond formation, the move of tRNA and mRNA on the ribosome, the dissociation of peptide chain, the dissociation of large and small subunits, the above studies are traced to a process in which ribosome synthesis is fixed. Ribosomal protein synthesis is a continuous dynamics process, which involves not only the ribosome itself, but also involves the synergy of ribosomal and translation factors. EF-G, a GTPase is involved in the second stage of protein synthesis - an extension of the process. tRNA and mRNA is constantly on the ribosome transfer to promote the extension of the nascent polypeptide chain in which EF-G and the energy molecule GTP are participated by the repeated binding and dissociation of EF-G and ribosome. How does EF-G play its role is still unknown.
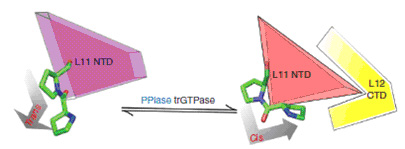 On 11 March 2012, Professor QIN Yan‘s group at the Institute of Biophysics, Chinese Academy of Sciences published an article in Nature Structural & Molecular Biology. This article, entitled "A conserved or proline switch on the ribosome facilitates the recruitment and the binding of trGTPases", reported a molecular mechanism of trGTPases integration and binding on the ribosome. QIN’s group found thatwhen elongation factor G (EF-G) binds to the ribosome, it first makes contact with the C-terminal domain (CTD) of L12 before interacting with the N-terminal domain of L11. They identified a universally conserved residue, Pro22 of L11, that functions as a proline switch (PS22), as well as the corresponding center of peptidyl-prolyl cis-trans isomerase (PPIase) activity on EF-G that drives the cis-trans isomerization of PS22. Only the cis configuration of PS22 allows direct contact between the L11 NTD and the L12 CTD. Mutational analyses of both PS22 and the residues of the EF-G PPIase center reveal their function in translational GTPase (trGTPase) activity, protein synthesis and cell survival in Escherichia coli. Their results demonstrated that all known universal trGTPases contain an active PPIase center. Their observations suggested that the cis-trans isomerization of the L11 PS22 is a universal event required for an efficient turnover of trGTPases, as part of the translation elongation cycle These studies have shown that L11 22 Proline is a very important regulatory site in the process of protein synthesis and provide a new drug target for antibiotic synthesis.
On 11 March 2012, Professor QIN Yan‘s group at the Institute of Biophysics, Chinese Academy of Sciences published an article in Nature Structural & Molecular Biology. This article, entitled "A conserved or proline switch on the ribosome facilitates the recruitment and the binding of trGTPases", reported a molecular mechanism of trGTPases integration and binding on the ribosome. QIN’s group found thatwhen elongation factor G (EF-G) binds to the ribosome, it first makes contact with the C-terminal domain (CTD) of L12 before interacting with the N-terminal domain of L11. They identified a universally conserved residue, Pro22 of L11, that functions as a proline switch (PS22), as well as the corresponding center of peptidyl-prolyl cis-trans isomerase (PPIase) activity on EF-G that drives the cis-trans isomerization of PS22. Only the cis configuration of PS22 allows direct contact between the L11 NTD and the L12 CTD. Mutational analyses of both PS22 and the residues of the EF-G PPIase center reveal their function in translational GTPase (trGTPase) activity, protein synthesis and cell survival in Escherichia coli. Their results demonstrated that all known universal trGTPases contain an active PPIase center. Their observations suggested that the cis-trans isomerization of the L11 PS22 is a universal event required for an efficient turnover of trGTPases, as part of the translation elongation cycle These studies have shown that L11 22 Proline is a very important regulatory site in the process of protein synthesis and provide a new drug target for antibiotic synthesis.