The world leading scientific journal Nature online published a breakthrough in lipopolysacharride biogenesisentitled"Structural basis for lipopolysaccharide insertion in the bacterial outer membrane"on Jun 18, 2014. The research team, lead by IBP structural biologist Yihua Huang, recently announced the structural determination of a membrane protein complex that is responsible for lipopolysaccharide export and assembly in Gram-negative bacterial outer membrane (OM).
"LPS is not only the main components of bacteria OM, but also the primary cause of innate immunity and inflammation responses in humans. Thus, the research on LPS biogenesis has been carried out over a hundred years and is fundamental importance.",says Prof. Yihua Huang, the senior author of the paper.
Lipopolysaccharide(LPS), also termed endotoxin, was first discovered by German renowned microbiologist Richard F. J. Pfeiffer at the end of nineteenth century.A hundred years later, American scientists Bruce Beutlerwon2011 Nobel Prize in Physiology or Medicine for identification of LPS receptor-Toll like receptor 4 in mammalian cells.LPS is synthesized in cytoplasm, and thereafter, flipped to the external leaflet of an inner membrane proteinflippase MsbA. The further export of LPS from periplasm to outer leaflet of OM was carried out by seven essential lipopolysaccharid transport proteins (LptA-F). Of them, the OM-localized LptD-LptEcomplex completed the final step of LPS biogenesis and, thus, represents an important drug target.
"The LptD-LptE complex structure is truly exciting. The high-resolution structure of the complex not only shed light on how LPS is transported across periplasm and finally assembled in the OM of Gram-negative bacteria, but also paved the way for developing new antibiotics against pathogens. ",says first-author ShuaiQiao, a graduate student in Professor Yihua Huang's laboratory.
"Yes, structural biologistshave many reasons to get excited about our structure.First, the complex structure reveals anunprecedented two-protein 'plug-and-barrel' architecture with LptE embedded into a 26-stranded barrelformed by LptD. Second, it is, for the first time,scientistsobserved two pairs of non-consecutive, inter-domain disulphide bonds in a membrane protein. Third, thebarrel formed by LptD is the most strand-containing and the largest βbarrel pore observed to date. Fourth, for the first time, we observed a β barrel with two strands adopting loop conformations. And, most importantly, we identified a potential LPS exit portal on the barrel wall, which gives clues how LPS is export into the lipid bilayer", says ProfessorXuejunCai Zhang, the co-author of the paper.
Currently, a group of peptidometic compounds based the structure of protegrinIhave been shown to target LptD, and a lead compound is active against the opportunistic pathogen P. aeruginosa. The crystal structure of the LptD/E complex willopen an avenue to new antibiotic strategies targeting the bacterial OM.
The work was supported by grants from the Ministry of Science and Technology, the Strategic Priority Research Program of the Chinese Academy of Sciences and the National Natural Science Foundation of China.
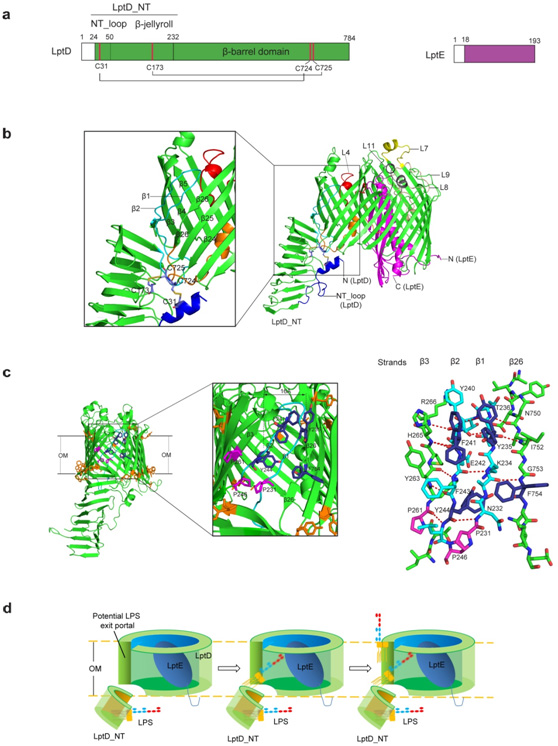
Fig1: TheLptD-LptE membrane protein complex crystal structure and model of LPS transfer and assembly at OM. a. Schematic structures of LptD and LptE; b. LptD-LptEmembrane protein complex crystal structure; c. Features of the LPS exit portal on the LptDbarrel wall; d. Proposed model for LPS insertion into bacterialouter membrane.