The famous international journal Genes & Development published a research article entitled “Structural transitions of centromeric chromatin regulate the cell cycle-dependent recruitment of CENP-N” on May 5, 2015. This article revealed an interesting mechanism of how CENP-A recruits CENP-N in the proper functioning of centromere. This article was brought by scientists from the Institute of Biophysics, Chinese Academy of Sciences.
The centromere is a specialized chromatin domain that provides a platform for kinetochore assembly and microtubule attachment, thus playing a crucial role in chromosome segregation during mitosis. Centromere-specific histone H3 variant, CENP-A, functions as an epigenetic mark for the formation and maintenance of centromeres.
CENP-N was identified as the first “reader” of epigenetic marks present in the CENP-A chromatin. Specific recognition of CENP-A chromatin by CENP-N is an essential process in the assembly of the kinetochore complex at centromeres prior to mammalian cell division. However, the mechanisms of CENP-N recruitment to centromeres and its function remain unknown.
IBP professor Li Guohong recently revealed the dual function of CENP-Ain the recruitment of CENP-N and the folding of centromeric chromatin. They found that a CENP-A-specific RG loop (Arg80/Gly81) plays an essential role in the recruitment of CENP-N. In addition, they found that CENP-A chromatin forms a featured “ladder-like” structure and the RG loop assists the formation of the “ladder-like” structure. Interesting, CENP-N specifically binds to open CENP-A chromatin and compaction of CENP-A chromatin impairs the binding of CENP-N.
Furthermore, they demonstrated that the higher-order organization of centromeric chromatin undergoes a structural transition from a compact state in G1 phase to an open state in S phase. They also verified that CENP-N is stably loaded in middle/late S phase. Interestingly, this structural transition of chromatin is consistent with the dynamic loading of CENP-N onto centromeres during the cell cycle. Thus they proposed that structural transitions of higher-order structure of centromeric chromatin orchestrate the temporal loading of CENP-N to centromeres via regulating the accessibility of the RG loop in CENP-A chromatin during the cell cycle.
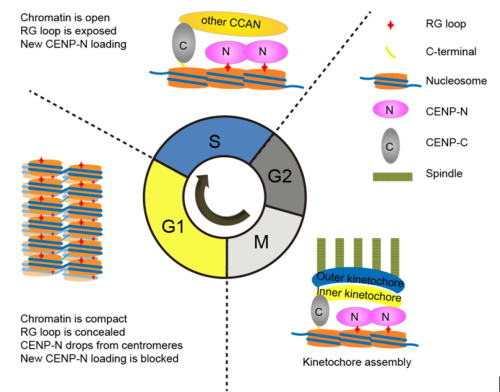
Legend: Model for structural transitions of centromeric chromatin regulating the cell cycle-dependent recruitment of CENP-N (From Fang et al. Genes & Development, 2015: Vol 29, no. 10)
This work provides critical insight and assists us to better understand the structure and function of centromeric chromatin, and how the epigenetic information encoded in CENP-A-containing nucleosomes is read and transmitted.
This study is supported by funds from the Ministry of Science and Technology of China, the National Natural Science Foundation, and the Chinese Academy of Sciences.
Link to the article: http://genesdev.cshlp.org/content/early/2015/05/04/gad.259432.115