Myocardial infarction (MI) is one of the leading causes of death especially among aging population. The incidence of MI rises every year. The detail mechanism of MI is complex and still not completely clarified. Expanding the knowledge of the molecular mechanisms underlying MI is of profound importance to developing a cure for MI.
Disruption of RNA metabolism at a variety of steps has emerged as a powerful and dynamic modifier of cardiovascular diseases. The CUG triplet repeat RNA-binding proteins (CUGBPs), a family of RNA-binding proteins, play key roles in RNA metabolism by regulating their splicing, stability and translation. Several studies have shown that CUGBP1 is an important regulator in the heart. However, the biological functions of CUGBP1 in human cardiovascular diseases remain poorly understood.
To clarity the roles and molecular mechanisms of CUGBPs in MI, Prof. JI Guangju and Dr. WANG Huiwen of the Institute of Biophysics (IBP) of the Chinese Academy of Sciences used an animal model of acute myocardial infarction (AMI) in their study.
In their research, they found that the CUGBP1 expression levels were decreased in heart samples from AMI mice, and further studies showed that two highly conserved AU-rich elements in the 3’UTR of CUGBP1 were responsible for the decreased CUGBP1 expression. Upon AMI, human antigen R was relocated to the cytoplasm from the nucleus and interacted with these AU-rich elements to disrupting the expression of CUGBP1. Reintroduction of CUGBP1 via gene delivery by recombinant adenovirus improved cardiac function in AMI mice. Their studies also indicated that CUGBP1 participated in the protection of cardiomyocytes from ischemia-induced injury through pro-angiogenic and anti-apoptotic pathways by controlling the vascular endothelial growth factor-A gene during AMI.
Their results provide the novel insights into the role of CUGBP1 in MI post-transcriptional gene regulatory machinery. The manipulation of CUGBP1 could be developed as a potential therapeutic option for the treatment of MI.
This study was published online in Antioxidants & Redox Signaling on March 28, 2017. The work was supported by the National Foundation of Sciences and Technology and SRF for ROCS.
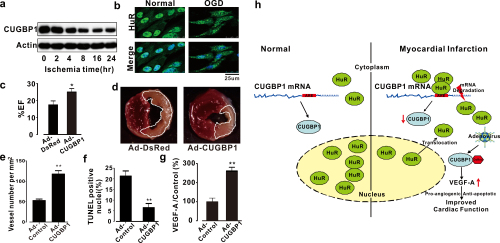
Figure: Reintroduction of CUGBP1 via gene delivery by recombinant adenovirus improved cardiac function in AMI mice (Image by IBP).
a, AMI leads to down-regulation of CUGBP1 expression. b, HuR was transported to the cytoplasm from the nucleus upon ischemia. c, Ultrasound assessment of EF at 4 weeks post-MI in mice expressing DsRed or CUGBP1. d, Infarct size analysis at 24 hr post-MI in mice expressing DsRed or CUGBP1. e, f, CUGBP1 protects AMI hearts by promoting angiogenesis (e) and inhibiting apoptosis (f). g, CUGBP1 regulates the VEGF-A gene in heart samples. h, Proposed mechanism of HuR-CUGBP1-VEGF-A regulation in AMI hearts.
Contact Person
Prof. JI Guangju
National Laboratory of Biomacromolecules
Institute of Biophysics, CAS
E-mail:gj28@ibp.ac.cn
Tel:86-10-64889873
or
Dr. WANG Huiwen
National Laboratory of Biomacromolecules
Institute of Biophysics, CAS
E-mail:whw@moon.ibp.ac.cn
Tel:86-10-64889873