The outer leaflet of the outer membrane (OM) of most Gram-negative bacteria primarily consists of lipopolysaccharide (LPS). The presence of LPS layer hampers the permeability of most hydrophobic antibiotics and allows them survive in a harsh environment. Transport of LPS across the cell envelope is carried out a multi-protein complex (Lpt), LptA-G that spans the double membranes of Gram-negative bacteria. Structural study of LptA-G complex or its sub-complexes is not only important for understanding how LPS, a typical amphipathic macromolecule, transports across the cell envelope, but also providing potential strategies for the design of novel antibiotics to inhibit its transport, because LPS is an essential component of the Gram-negative OM. In 2014, Prof.HUANG Yihua’s group at Institute of Biophysics, Chinese Academy of Sciences, determined the crystal structure of OM-localized two-protein complex, LptD-LptE from Shigella flexneri. This work provided important mechanistic insights in the LPS transport across the OM and its insertion in the external leaflet of the OM (Nature 2014, http://www.nature.com/nature/journal/v511/n7507/full/nature13484.html). To further understand how LPS is extracted from the inner membrane (IM), Prof. HUANG’s group recently solved the crystal structure of LptB2FG, an unusual ABC transporter that is response for selectively extracting LPS from outer leaflet of the IM. The structure of LptB2FG revealed that LptF and LptG each contain a transmembrane domain (TMD) consisting of six transmembrane helices (TM), a periplasmic β-jellyroll-like domain and a coupling helix that interacts with each copy of the dimeric nucleotide-binding domain (NBD) LptB on the cytoplasimic side. The two TMDs of LptF and LptG arrange to form a large outward-facing “V”-shaped cavity in the inner membrane (IM) via limited interactions between LptF and LptG. Structure-based in vivo analyses suggest that LPS may enter into the central cavity laterally via the interfaces of the two TMD domains and is further expelled into the β-jellyroll-like domains upon ATP binding and hydrolysis. Unlike most ABC transporters that transport their substrates across the IM, LptB2FG extracts LPS from the outer leaflet of the IM and delivers it to the periplasmic component LptC, representing a new type ABC exporters — Type III ABC exporter, distinct from Type I and Type II ABC exporters. The work has been recently published online in Nature Structural & Molecular Biology on April 10, 2017. The research was supported by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. 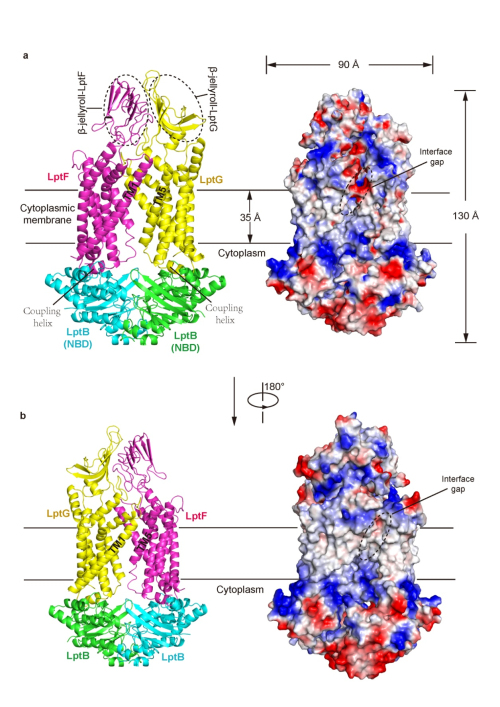
Figure: Crystal structure of LptB2FG complex (Image by IBP) Contact HUANG Yihua, Principle Investigator National Laboratory of Biomacromolecules Institute of Biophysics, Chinese Academy of Sciences Tel:86-10- 64888789 (office) E-mail: yihuahuang@sun5.ibp.ac.cn |