The study entitled “Structural basis for GTP hydrolysis and conformational change of MFN1 in mediating membrane fusion” was published on line in the journal of Nature Structural &Molecular Biology on February 26, 2018. This work determinedthe dimer structure of the minimal GTPase domain (MGD) of human MFN1 in complex with GDP-BeF3-.The findingssuggest that a swing of the four-helix bundle induced by GTP hydrolysis can pull tethered membranes closer to achieve fusion. It also offerscritical explanation forhow Charcot-Marie-Tooth neuropathy type 2A (CMT2A)-causing mutations compromise MFN-mediated fusion.
Mitochondria undergo constant fusion and fission tomaintain proper function. The outer mitochondrial membrane is merged by a class of dynamin-like, membrane-bound GTPases, including MFN1 and MFN2 in mammalsand Fzo1p in yeast. Deletion of MFN causes mitochondrial fragmentation in cells and embryonic lethality in mice. Mutations inhuman MFN2 have been linked to CMT2A. However,the mechanism by which the mitochondrial membranes fuse is not clear.
Based on previous work (JCB, 2016), the structure of MGD, in the form of a dimer in the presence of GDP and BeF3-, was determined at 3.2Åresolution. Notably, thenew MGD dimer buries a larger surface area at the interface thanthe previously reported MGD dimer and the HB1 domains are almost parallel and point in the samedirection.This conformation is designated as the ‘HB-closed’state. Conversely, the HB1s in the previously reported MGDdimer point in opposite directions as an ‘HB-open’ state.An updated model of MFN-mediated fusion is proposed, using a combination of structural analysis, in vitrobiochemical assays and mitochondria morphology rescue assays in MFN1-deleted cells. In addition, it is revealed that GTP hydrolysis by MFN1 requires potassium ion.
The proposed mechanism involves drasticconformational changes in interacting MFN molecules andprovides new insights intohow MFN mediates homotypic membrane fusion. It also explainedthe effect of mutations in MFN2 that cause CMT2A. Same mechanism can be applied to MFN2 and the yeast homolog Fzo1p.
Prof. Junjie Hu at the Institute of Biophysics (IBP) of the Chinese Academy of Sciences (CAS) and Prof. Zhiyong Lou at Tsinghua University are co-corresponding authors.Liming Yan and Caiting Yu from Prof. ZiheRao’s team and Yuanbo Qi and Xiaofang Huang from the Hu group share the first authorship. This work was supported by theNational Key Research and Development Program, the National Natural Science Foundation of China and anInternational Early Career Scientist grant from Howard Hughes Medical Institute.
Article link: https://www.nature.com/articles/s41594-018-0034-8
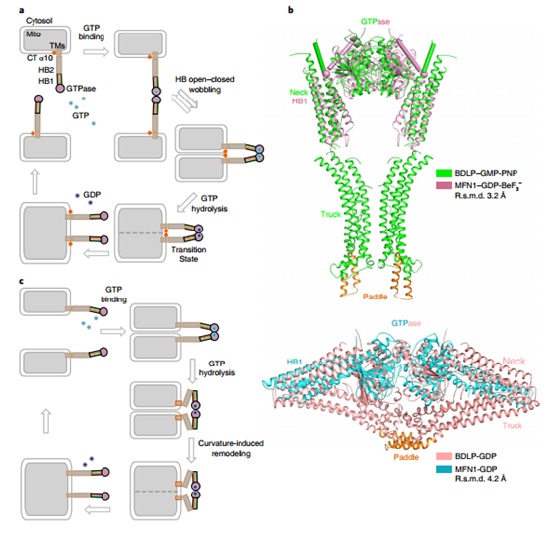
Figure: The proposed model of MFN-mediated fusion.
By Junjie Hu reserach group
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64886852
Email: huj@ibp.ac.cn