The research work entitled “Cryo-EM structure of a herpesvirus capsid at 3.1 Å” was published on line in the journal of Science on April 6, 2018. Using a combination of “block-based” reconstruction and accurate Ewald sphere corrections, researchers have reconstructed the 3.1 Å structure of HSV-2 B-capsid and built the atomic model, which extends our understanding of the assembly mechanism of the capsid.
The herpesvirus, genetically as well as structurally one of the most complex viruses, spreads within the host population efficiently, causing a range of diseases in human, including congenital disorders and cancers. As one of the four major structural layers, the 125 nm-capsid of herpesvirus not only protects viral genome from mechanical and other damage, but also functions to release the viral genome into the host cell nucleus in the initial infection and package the genome again in the maturation.The assembly pathway of herpesvirus produces three distinct types of capsids called A-, B- and C-capsids respectively. All the three types of capsid have mature angular shells and share with the same structure proteins and the similar assembling mechanism. However, little is known on the structure and the assembly mechanism of the HSV capsid.
The researchers found that there are four major conformers of the major capsid protein, VP5, which exhibit striking differences in configuration and mode of assembly to form extensive intermolecular networks. The triplex, a heterotrimeric assembly that fits between hexamers and pentamers at quasi-three-fold positions to cement the capsid together, consists of two copies of VP23, each exhibiting remarkably different conformations, and one copy of VP19C. Six copies of the small capsid protein, VP26, form a ring on the top of the hexon and further stabilize the capsid.
Basing on the capsid structure, the researchers proposed a model for the ordered assembly of the capsid using basic assembly units (a triplex and its covalently linked lasso triangle formed by three VP5s), which then cluster into higher-order structures conforming to two fold symmetry and guide nascent assembly intermediates into the correct T = 16 geometry, allowing the first steps toward understanding the drivers of assembly and the basis of stability of the capsid.
Professor WANG Xiangxiand Professor Zhang Xinzheng at the Institute of Biophysics, Professor Liu Hongrong at the Hunan Normal University, Professor Wang Junzhi at the National Institutes for Food and Drug Control, in cooperation with Professor RAO Zihe are corresponding authors.The study was supported by the National Key Research and Development Program, the Strategic Priority Research Program, National Science Foundation of China.
Artical link: http://science.sciencemag.org/content/360/6384/eaao7283
Artical commentory: 饶子和团队等揭示疱疹病毒的组装机制——施一、张凯点评
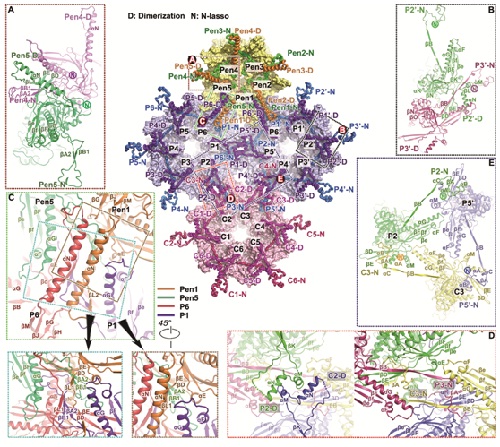
Overview of the interactions at inner capsid surface.
Contact: WANG Xiangxi
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64858554
Email: xiangxi@ibp.ac.cn