The endoplasmic reticulum (ER) forms membrane contact sites (MCSs) with multiple organelles, including the mitochondria, Golgi, lipid droplets (LDs) and endosomes. Establishing an MCS involves the formation of a tethering complex that simultaneously binds to the two apposing membranes. Autophagy involves the engulfment of a portion of the cytosolic contents in a double-membrane autophagosome and its subsequent delivery to the lysosome for degradation. Autophagosome formation involves the nucleation of a cup-shaped membrane sac, known as the isolation membrane (IM), and its further expansion and closure. IMs form extensively contacts with the ER during expansion. The molecular mechanisms underlying establishment and maintenance of the ER/IM contact are poorly understood.
Recently, a study led by Prof. Hong Zhang from the institute of Biophysics (IBP) of the Chinese Academy of Sciences (CAS) was reported in Current Biology, entitled "The ER contact proteins VAPA/B interact with multiple autophagy proteins to modulate autophagosome biogenesis". This study reveals that VAPs directly interact with multiple ATG proteins, thereby contributing to ER/IM contact formation for autophagosome biogenesis.
The integral ER proteins VAPA and VAPB (VAPs) participate in establishing ER contacts with multiple membranes by interacting with different tethers. In this study, the authors determined whether VAPs also act in the autophagy pathway. By performing a series of assays, including the conversion of LC3, degradation of the autophagy substrate p62 and ultrastructural analysis, the authors found that depletion of VAPA/B impairs the progression of IMs into autophagosome. Further analysis indicated that VAPA/B directly interact with FIP200 and ULK1 through their conserved FFAT motifs and stabilize the ULK1/FIP200 complex at the autophagosome formation sites on the ER. VAPA/B also interact with WIPI2 and enhance the formation of the WIPI2/FIP200 ER/IM tethering complex. Depletion of VMP1, which increases the ER/IM contact, greatly elevates the interaction of VAPA/B with these autophagy proteins. The VAPB P56S mutation, which is associated with amyotrophic lateral sclerosis, reduces the ULK1/FIP200 interaction and impairs autophagy. This study reveals that VAPA/B directly interact with multiple ATG proteins, contributing to ER/IM contact formation for autophagosome biogenesis.
This study was supported by the grants from the National Basic Research Program of China, the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS), and the Key Research Program of Frontier Sciences, CAS.
Article link: https://www.cell.com/current-biology/fulltext/S0960-9822(18)30303-8
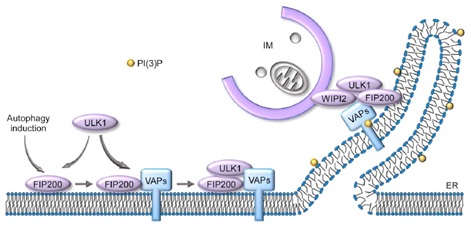
Figure: Model of VAPA/B function in autophagy.
At the initiation step of autophagosome formation, FIP200 recruits VAPs to the autophagosome formation sites, which further recruits or stabilizes ULK1 to FIP200 puncta. VAPs also participate in the formation and/or stabilization of the ULK1/FIP200-WIPI2 tethering complex to mediate ER/IM formation during IM expansion.
Contact: Zhang Hong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64848238
Email: hongzhang(AT)sun5.ibp.ac.cn