Semaphorins are a large family of secreted and membrane bound glycoproteins involve in axon guidance, neural development, angiogenesis, bone differentiation and cardiovascular development. Some of them are named as Immune Semaphorins due to their regulation of immune responses. Mouse Semaphorin-4A (mSema4A) has been implicated in the co-stimulation of T cells and drives Th1 immune responses by binding to the receptor, T-cell immunoglobulin and mucin domain protein 2 (Tim-2) to produce IL-2. However, there is no human ortholog of Tim-2. So far, the biological functions of Sema4A in human immune system are unknown.
In a study published in Nature Communications, the researchers at Institute of Biophysics of Chinese Academy of Sciences (CAS) in Beijing disclosed that, unlike mouse Sema4A, which preferentially induces Th1 immune responses, human SEMA4A (hSEMA4A) induces robust Th2 responses. The receptor for hSEMA4A was identified as immunoglobulin-like transcript 4 (ILT-4).
The researchers found that hSema4A is preferentially expressed on antigen-presenting cells and CRTH2+ Th2 memory cells, it co-stimulates T cells strongly. In Th2 culture condition, hSema4A up-regulates CD4+T cells producing big amount of Th2 cytokines including IL-4, IL-5 and IL-13.
They also revealed that hSema4A enhance GATA3 expression and STAT6 activation and inhibit T-bet expression during CD4+T cells differentiation.
By employing two independent cloning strategies, it was demonstrated that Immunoglobulin-like transcript 4 (ILT-4) is a receptor for human SEMA4A (hSEMA4A) on activated CD4+T cells.
Furthermore, in human asthmatic lung tissue, both hSEMA4A and its receptor ILT-4 were highly expressed, implying its potential function in disease pathogenesis.
This study defined a different biological function of hSEMA4A from its murine homolog through its binding to the receptor of ILT-4 to co-stimulate CD4+T cells and regulate Th2 cells differentiation.
The study was supported by the following scientists world round:
Dr. Yong-jun Liu (M. D. Anderson Cancer Center, The University of Texas; Oversea group in CAS Key Laboratory of Infection and Immunity, Institute of Biophysics),Dr. Zhiqiang Zhang (Immunobiology and Transplant Research, Houston Methodist Hospital and Methodist Hospital Research Institute),Dr. Lieping Chen (Yale University School of Medicine; Oversea group in CAS Key Laboratory of Infection and Immunity, Institute of Biophysics),Dr. Ying Sun (King’s College London; School of Basic Medical Sciences, Capital Medical University) , Dr. Sheng Yao (Top Alliance Biosciences) and Dr. Shengdian Wang (Institute of Biophysics, Chinese Academy of Sciences).
Article link:https://www.nature.com/articles/s41467-018-03128-9
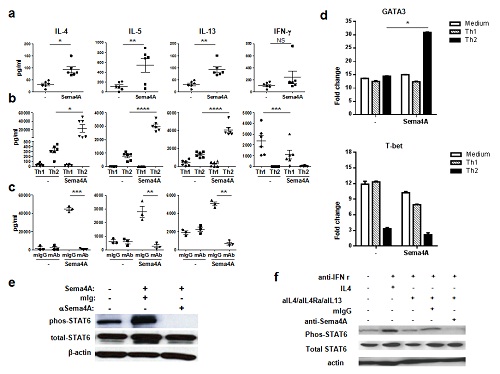
Fig.1 Sema4A enhances Th2 polarization of Th2-primed CD4+ T cells.
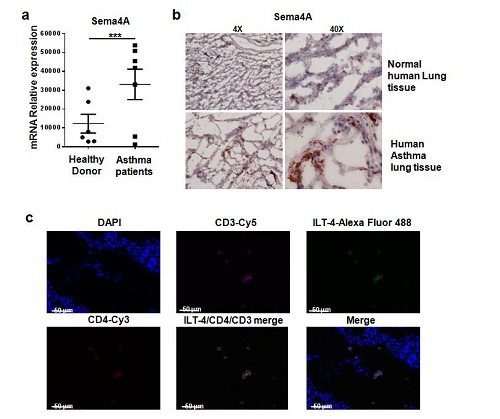
Fig.2 Sema4A and ILT4 are specifically over expressed in human asthma lung tissue.