Colorectal cancer (CRC) is the third most common type of cancer globally. CRC progresses through an adenoma-to-carcinoma sequence that eventually leads to metastasis, preferentially (~70% patients) to the liver. At this phase, the disease becomes challenging to treat and eventually develops resistance to most forms of combination therapy, making CRC metastasis a leading cause of cancer-related deaths.
Current chemotherapy for advanced CRC does not target liver metastases specifically. This is partly based on observations that CRC metastases are not consistently associated with any specific genetic mutations and that they generally resemble cells in the primary tumor. However, emerging evidence suggests that non-genetic alterations, such as epigenetic and metabolic reprogramming, may promote cancer metastasis. Targeting such mechanisms may provide a new way to develop therapeutics against metastasis.
In a study published in cell metabolism, the researchers at Institute of Biophysics of Chinese Acad-emy of Sciences (CAS) and at Department of Biomedical Engineering of Duke Universi-ty investigated that aldolase B-mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis, and targeting aldolase B or reducing dietary fructose reduces liver metastasis growth.
The researchers found specific metabolic pathways are altered in CRC liver metastases by utilizing data from both clinical samples and an in vivo CRC metastasis model via cecum transplantation. In particular,liver metastases upregulate ALDOB, an enzyme involved in fructose metabolism. Intra-hepatic implantation indicates that the liver environment causes CRC cells to upregulate ALDOB.
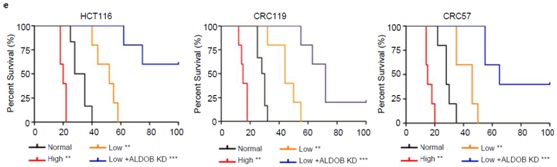
They also revealed that ALDOB promotes fructose metabolism to fuel glycolysis, gluconeogenesis, and the pentose phosphate pathway by metabolomics and 13C-labeled fructose tracing studies. ALDOB knockdown or dietary fructose restriction suppresses growth of CRC liver metastases but not primary tumors or lung metastases, highlighting the importance of tumor microenvironment.
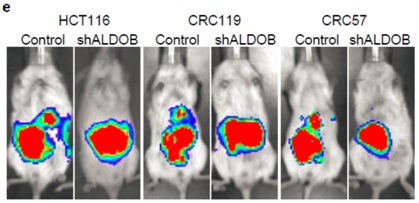
Silencing of ALDOB suppresses CRC liver metastasis
Dietary fructose restriction suppresses CRC liver metastasis
This study highlighted that metastatic cells can take advantage of reprogrammed metabolism in their new microenvironment, especially in a metabolically active organ such as the liver. Targeting ALDOB and fructose metabolism has the potential to affect the growth of liver metastases and to complement current chemotherapies.
This work was partly supported by the National Basic Research Program of China,Strategic Priority Research Program of the Chinese Academy of Sciences.
Article link: https://www.cell.com/cell-metabolism/fulltext/S1550-4131(18)30244-4
Contact:BU Pengcheng
Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China
Phone: 86-10-64889588
Email: bupc(AT)ibp.ac.cn