A research entitled “Secretory kinase Fam20C tunes endoplasmic reticulum redox state via phosphorylation of Ero1a” was published online in the EMBO Journal on Jun.1st, 2018. Prof. WANG Chih-chen’s group reports that secretory kinase Fam20C catalyzes the phosphorylation of Ero1a tomaintain endoplasmic reticulum (ER) redox balance and promote oxidative protein folding.This study opens a door to uncover how oxidative protein folding is regulated by phosphorylation in the secretory pathway.
Protein phosphorylation regulates almost every aspect of cell life. It is estimated that perhaps 30% of human proteins are phosphoproteins. Over the past few decades, researches have been focused on elucidating protein phosphorylation regulation in the cytoplasm and nucleus. Although the first phosphoprotein, casein, was reported in 1883, family with sequence similarity 20C (Fam20C) - the bona fide kinase catalyzing the phosphorylation of secreted protein casein - was not identified until 2012. Loss-of-function mutations in Fam20C cause a rare and often lethal osteosclerotic bone dysplasia called Raine syndrome. Fam20C is the kinase responsible for generating the majority of secreted phosphoproteome (more than 100 secreted proteins) implicated in a broad spectrum of biological processes. However, little is known about the role of protein phosphorylation in the lumen of the secretory pathway.
Prof. WANG Chih-chen’s group works on the mechanisms of disulfide bond formation and redox homeostasis maintenance in the ER. In this study, they have identified a total of 349 proteins localized in the ER and Golgi apparatus that specifically interact with Fam20C. Notably, enzymes responsible for oxidative protein folding are enriched in the Fam20C interactome. Using a super folded ER-localized redox-sensitive green fluorescent protein (superfolded-roGFP-iEER), a more reduced ER is observed in FAM20C knockout cells. Further, researchers find that Ero1α, the pivotal sulfhydryl oxidase that catalyzes disulfide formation in the ER, is phosphorylated by Fam20C at Ser145. Phosphorylation on Ser145 greatly enhances Ero1α oxidase activity both in vitro and in vivo, which is a novel activity controller independent of the known “regulatory disulfide switch” in Ero1α. Moreover, Ero1α is phosphorylated by Fam20C in the Golgi and retained in the ER by ERp44/KDELR. This observation also provides an answer to the long-unsolved question of why Ero1α functions in the ER but does not contain an ER retention motif. Interestingly, phosphorylation of Ero1α is induced under hypoxia, reductive stress and secretion-demanding conditions such as mammalian lactation, suggesting that phosphorylation of Ero1α by Fam20C plays an important physiological role.
Associated Prof. WANG Lei (from Prof. WANGChih-chen’s group) is the corresponding author. Prof. XIAO Junyu provides great support in this study. ZHANG Jianchao from Prof. WANG’s group is the first author. The study was supported by the National Key R&D Program of China, National Natural Science Foundation of China and Youth Innovation Promotion Association CAS.
Article link: http://emboj.embopress.org/content/early/2018/06/01/embj.201798699
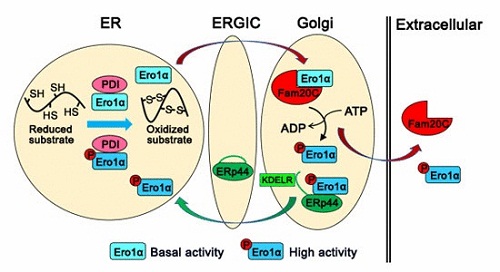
A model illustrating how Fam20C tunes ER redox homeostasis and oxidative protein folding
Contact: WANG Lei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China