Chinese Academy of
Sciences Protein
Science core facility
Center |
|
|
Structure of state-transition supercomplex PSI-LHCI-LHCII reveals the adaptation and regulation mechanisms of plant during photosynthesis |
| Author: |
|
Update time: |
2018-06-08 |
|
Photosynthesis is one of the most amazing chemical reactions, providing food and energy for almost all living organisms in the planet. In addition, oxygenic photosynthesis is crucial for the formation of the atmosphere and maintenance of the carbon-oxygen balance on the earth. The primary photochemical reaction is accomplished mainly by four membrane-embedded supramolecular machineries, including photosystems I and II (PSI and PSII). The light-driven electron transport is fulfilled by the two photosystems, and their energy transfer efficiency is higher than 90%.
In the natural environment, the light intensity and quality are constantly changing. The antenna systems of PSI or PSII have different pigment composition and hence different light absorption properties. Thus fluctuating illumination can cause unequal excitation of the two photosystems. Balanced light harvesting is crucial for efficient photosynthesis, and plants have evolved sophisticated regulatory mechanisms in order to optimize the photosynthetic efficiency and to avoid photo-damage. State transitions are one of the short-term adaptation mechanisms and necessary for optimal plant growth and fitness in the field. Analysis on the regulatory mechanism of photosynthesis is not only of great significance in basic research, but also has application prospect.
During state transitions, the trimeric LHCII is reversibly phosphorylated and de-phosphorylated, and migrates between the two photosystems. Under light conditions favoring PSII excitation, over-excitation of PSII leads to the activation of LHCII kinase and subsequent phosphorylation of the N-terminal region of LHCII. A portion of the phosphorylated LHCIIs (mobile LHCII) move laterally within the thylakoid membrane from PSII to PSI, forming the PSI-LHCI-LHCII supercomplex. The mobile LHCII serves as a peripheral antenna of PSI in addition to LHCI, increasing energy transfer towards PSI core. Structural analysis of the PSI-LHCI-LHCII supercomplex is a pivotal step for better understanding of the subunit arrangement, the inter-molecular interaction between PSI and LHCII, the potential energy transfer pathways within the supercomplex.
CHANG Wenrui - LI Mei’s group and ZHANG Xinzheng’s group from the Institute of Biophysics (IBP) at CAS collaborate and solved the cryo-electron microscopy (cryo-EM) structure of PSI-LHCI-LHCII supercomplex from maize at 3.3 Angstrom resolution. The supercomplex has a total molecular mass of ~700 kilodalton. Total of 21 protein subunits, 202 chlorophylls, 47 carotenoids and numerous other cofactors were identified in the final structure. In this work, for the first time, the phosphorylation site in LHCII was solved, the detailed interactions between LHCII and PSI was revealed. In addition, they further solved the structure and location of two PSI core subunits (PsaN and PsaO), which are absent in the previously reported crystal structures. The structure showed that PsaN and PsaO are at the PSI-LHCI interface and the PSI-LHCII interface, respectively. Each subunit relays excitation to PSI core through a pair of chlorophyll molecules, thus revealing previously-unseen paths for energy transfer between the antennas and the PSI core.
The research work, entitled “Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II”, was published in Science on Jun. 8, 2018. The breakthrough is achieved through continuous and persistent efforts by the team from IBP after their previous works on the cryo-EM structure of plant PSII-LHCII supercomplexes (Nature, 2016; Science, 2017). The project was supported by grants from the Chinese Academy of Sciences, the Ministry of Science and Technology of China, the National Natural Science Foundation of China. http://dx.doi.org/10.1126/science.aat1156 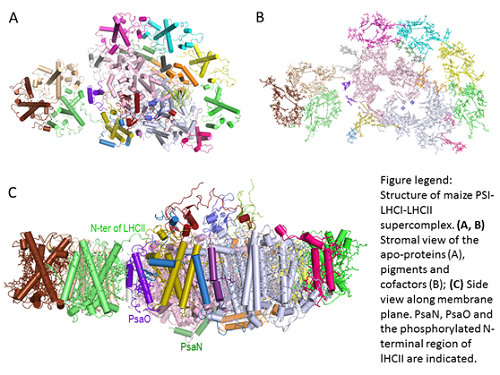
Contact: Mei Li
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: meili(AT)ibp.ac.cn
|
|
|
|