In mammals, females have a limited supply of oocytes. These oocytes also have a unique epigenome with approximately half the DNA methylation of sperm and most terminally differentiated somatic cells. Until recently, regulators of this unique DNA methylation pattern and its functional significance were unknown.
Now, a novel DNA methylation regulator Stella, whose ectopic overexpression in somatic cells led to global DNA demethylation through disrupting the function of the DNA methylation regulator UHRF1, has been identified.
In a recent study published online ahead of print in Nature, a joint research group led by Dr. ZHU Bingfrom the Institute of Biophysics of the Chinese Academy of Sciences reveals that Stella sequestered UHRF1 from the nucleus through an active nuclear export process, and the dysregulation of UHRF1 by loss of Stella resulted in an accumulation of aberrant DNA methylation during postnatal oogenesis.
These findings show the first regulatory factor found to safeguard the unique methylation status of the oocyte genome.
Since Stella is highly expressed in oocytes, the researchers focused on the in vivo function of Stella during oogenesis.
Earlier studies revealed that Stella-null oocytes were incapable of supporting the development of preimplantation embryos. This study shows preferential hypermethylation at the transcriptionally inert regions of Stella null oocytes.These aberrant promoters of hypermethylation on the maternal allele severely affected zygotic genome activation and development of the preimplantation embryo.
Interestingly, a maternal genome lacking DNA methylation hasd been reported tonot affect preimplantation embryo development, while this study suggests that keeping a uniquely hypomethylated oocyte genome is vital.
Moreover, researchers found that DNMT1, generally considered to be a maintenance DNA methyltransferase, which is only active on hemi-methylated DNA in vivo, is the major DNA methyltransferase responsible for the aberrant DNA methylation in Stella-deficient oocytes and unambiguously proves the de novo methylation activity of DNMT1 in vivo.
This discovery rewrites the textbook classification of DNA methyltransferases. Also, it sheds light on a functional role of DNMT1 in post-mitotic cells, which may help to reveal a role for DNMT1 in ageing.
The study was supported by the China National Science Foundation, the Chinese Ministry of Science and Technology, the Chinese Academy of Sciences, and the Science and Technology Commission of Shanghai, among others. 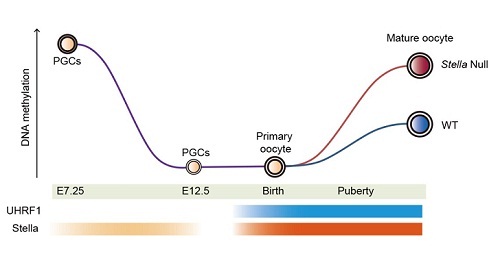
Stella sequesters UHRF1 to safeguard the unique methylome of oocytes. (Image by Dr. ZHU Bing’s group)
Article link: https://www.nature.com/articles/s41586-018-0751-5 Contact: Bing Zhu
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101,China
Phone: 86-10-64888832
Email: zhubing@ibp.ac.cn (Reported by Dr. ZHU Bing’s group) |