Chinese Academy of
Sciences Protein
Science core facility
Center |
|
|
The multifunctional human p100 protein "hooks" ethylated ligands |
| Author: |
|
Update time: |
2009-11-30 |
|
|
|
Important achievements
|
|
The Multifunctional Human P100 Protein "Hooks" Ethylated Ligands
Nat Struct Mol Biol 2007 ,14(8):779-784 |
|
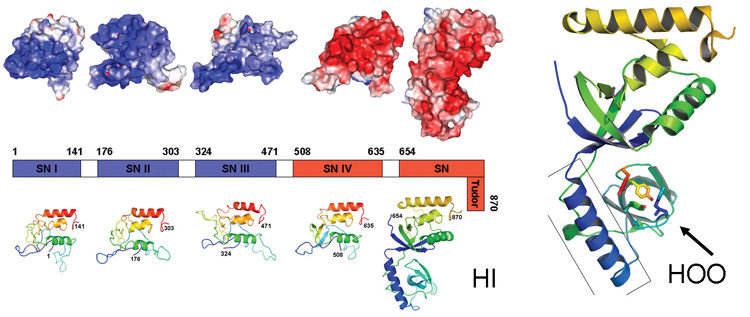
Liu Zhijie's group solved the detailed 3D structure of human p100 protein, a vital regulator of cellular transcription processes. p100 has been shown to act as a physical bridge between promoter-specific activators and the basal transcription machinery, resulting in an increase in the level of gene transcription. They demonstrated interaction between the tudor domain of p100 and the U snRNP complex that suggested a role for p100 in the processing of pre-mRNA. The crystal structure of the p100 SN-Tudor domain was determined in order to delineate the molecular basis of the proposed functions of p100. The interdigitated structure resembled a hook, with a hinge controlling the movement and orientation of the hook. Their studies suggested that a conserved aromatic cage hooks methyl groups of snRNAs and anchors p100 to the spliceosome. Such findings provide important structural insights that partly explain the distinct roles of p100 in transcription and splicing.
|
|
|
|
|
|
|