
Zhenfeng Liu, Ph.D, Prof.
-
Principal Investigator, IBP Deputy Director
State Key Laboratory of Biomacromolecules, IBP
Research Interests: Structure and function of membrane proteins
Email: liuzf@ibp.ac.cn
Tel: 010-64881481 (office), 010-64889535 (lab)
Address: 15 Datun Road, Chaoyang District, Beijing, 100101, China
Chinese personal homepage
- Biography
1998 - 2004 Doctor of Science in Biophysics, August, 2004, Instiute of Biophysics, Chinese Academy of Sciences Beijing, China
1994 - 1998 Bachelor of Science in Biology, July, 1998, Department of Biology, Xiamen University, Fujian Province, China
Employment history
2004.12 - 2010.12 Research Associate, Howard Hughes Medical Institute, Postdoctoral Scholar Division of Chemistry and Chemical Engineering California Institute of Technology, Pasadena, California, USA
2011.01 - Investigator, Institute of Biophysics, Chinese Academy of Sciences, Beijing
- Awards
- Membership in Academies & Societies
- Research Interests
Membrane proteins have fundamental roles in energy absorption and conversion during photosynthesis, electron transfer and oxidative phosphorylation during respiration process, transport of substances across the cellular membrane, signal transduction and catalysis of intramembrane enzymatic reactions such as proteolysis and lipid biosynthesis/hydrolysis. It was estimated that 20-30% of the open reading frames in both prokaryotic and eukaryotic genomes encode membrane proteins, underscoring the importance of membrane protein research. So far, membrane protein structures only account for ~1% of the total protein structures deposited in the Protein Data Bank (PDB), indicating the field of membrane protein structural research largely lags behind that of soluble proteins. This is mainly due to the challenge in heterologous overexpression, purification and crystallization of membrane proteins. Nevertheless, membrane protein structural biology is becoming a leading-edge branch of structural biology and attracts more and more researchers to join and develop this field which is still at its early stage of exponential growth (http://blanco.biomol.uci.edu/Membrane_Proteins_xtal.html).
My laboratory studies structure and function of membrane proteins from different biological systems, using (but not limited to) the techniques of X-ray crystallography and Cryo-electron Microscopy. The targets include the bacterial mechanosensitive channels involved in osmotic pressure regulation, intramembrane enzymes catalyzing phospholipid biosynthesis and remodeling and membrane protein complexes related to photosynthetic state transition. The goal is to elucidate the molecular mechanism of these fundamental biological processes through in-depth structural and functional analyses.
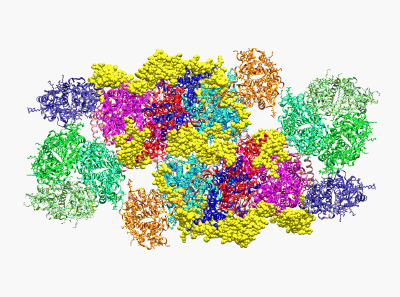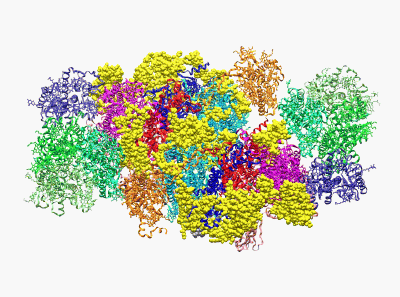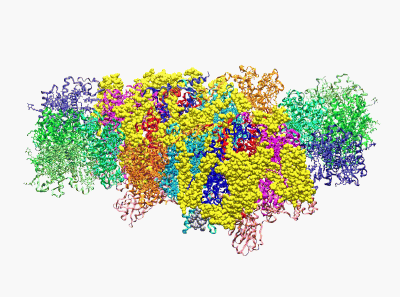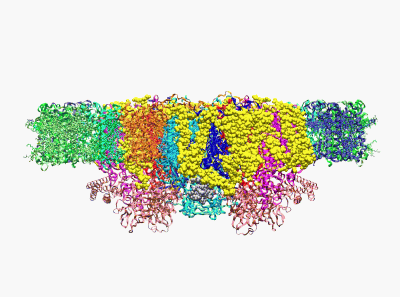
(Wei X. P., Su X. D., et al. Nature 2016)
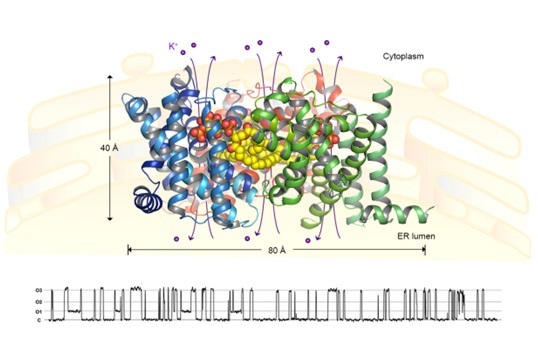
(Yang H.T., Hu M.H., et al. Nature 2016)
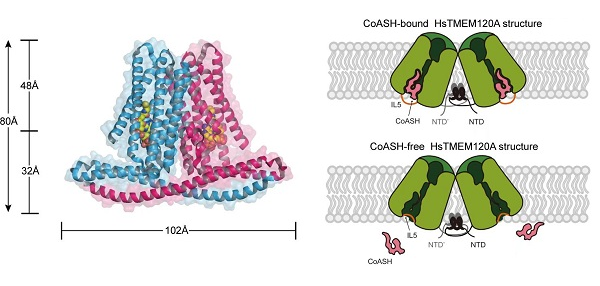
Figure The TMEM120A protein involved in lipid metabolism contains a coenzyme A (CoASH)-binding site.
Left: Overall architecture of HsTMEM120A homodimer in complex with CoASH molecules.
Right: Conformational change of TMEM120A induced by CoASH molecule.
(Rong, Y., Jiang J., Gao Y., et al. Elife 2021)
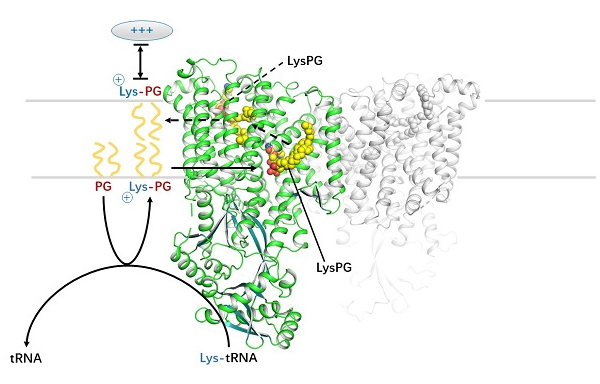
The mechanism of aminoacyl phospholipid biosynthesis and translocation mediated by MprF and its relevance with the resistance of bacteria to cationic antimicrobial peptides.
(Song, D., Jiao H. & Liu Z. Nat. Commun. 2021)
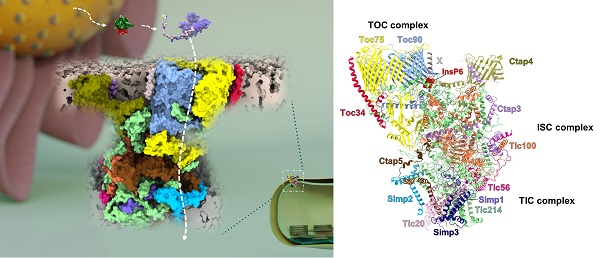
Architecture and subunit arrangement of chloroplast TOC-TIC translocon supercomplex
(Liu H. et al. Nature 2023)
- Grants
- Selected Publications
1. Liu, X.Y.# *, Li, J.J. #, Song, D.F. and Liu, Z.F.*. Structural mechanisms underlying the free fatty acid-mediated regulation of DIACYLGLYCEROL O-ACYLTRANSFERASE 1 in Arabidopsis. The Plant Cell, (2025). DOI: https://doi.org/10.1093/plcell/koaf239
2. Caferri, R.#, Zhou, Q.#, Dall'Osto, L., Amelii, A., Shan, J.Y., Liu, Z.F. and Bassi, R*. A stress-induced paralog of Lhcb4 controls the photosystem II functional architecture in Arabidopsis thaliana. Nat. Commun. 16: 6910 (2025). DOI: 10.1038/s41467-025-62085-2.
3. Wang, Y.D., Wang, C.X., Li, A.J. and Liu Z.F.*. Roles of multiple TEF30-associated intermediate complexes in the repair and reassembly of photosystem II in Chlamydomonas reinhardtii. Nat. Plants doi: 10.1038/s41477-025-02036-3. (2025). Research Briefing:Structural snapshots show the roles of TEF30 in repairing the broken photosystem II. Article link: https://rdcu.be/ewkUG.
4. Shan J.Y., Niedzwiedzki, D.M., Tomar, R.S., Liu, Z.F.*, Liu H.J.*. Architecture and functional regulation of a plant PSII-LHCII megacomplex. Sci. Adv., 10: adq9967 (2024). DOI: 10.1126/sciadv.adq9967
5. Li A.J., You T.T., Pang, X.J., Wang Y.D., Tian L.J., Li X.B*. and Liu Z.F*. Structural basis for an early stage of the photosystem II repair cycle in Chlamydomonas reinhardtii. Nat. Commun., 15: 5211 (2024) DOI: 10.1038/s41467-024-49532-2. (To read the article, click https://rdcu.be/dLbRp)
6. Ishii A., Shan J.Y., Sheng X., Kim E., Watanabe A., Yokono M., Noda C., Song C.H., Murata K., Liu Z.F.* and Minagawa J.*. The photosystem I supercomplex from a primordial green alga Ostreococcus tauri harbors three light-harvesting complex trimers. Elife, 12:e84488.( 2023) DOI: 10.7554/eLife.84488.
7. Liu, H., Li A.J., Rochaix, J.-D.and Liu Z.F.*. Architecture of chloroplast TOC-TIC translocon supercomplex. Nature, (2023). DOI: https://doi.org/10.1038/s41586-023-05744-y
8. Yang, B.W., Yao H.B., Li D.F. and Liu Z.F.*. The phosphatidylglycerol phosphate synthase PgsA utilizes a trifurcated amphipathic cavity for catalysis at the membrane-cytosol interface. Current Research in Structural Biology, in press (2021), DOI: https://doi.org/10.1016/j.crstbi.2021.11.005
9. Rong Y., Jiang J.H., Gao Y.W., Guo J.L., Song D.F., Liu W.H., Zhang M.M., Zhao Y.*, Xiao B.L.*, Liu Z.F.*. TMEM120A contains a specific coenzyme A-binding site and might not mediate poking- or stretch-induced channel activities in cells. Elife, 10, e71474 (2021).
10. Pan X.W., Tokutsu R., Li A.J., Takizawa K., Song C.H., Murata K., Yamasaki T., Liu Z.F.*, Minagawa J.* and Li M.*. Structural basis of LhcbM5-mediated state transitions in green algae. Nat. Plants,(2021) , DOI: https://doi.org/10.1038/s41477-021-00960-8
11. Sheng X., Liu Z.F.*, Kim E.,and Minagawa J.*. Plant and Algal PSII–LHCII Supercomplexes: Structure, Evolution and Energy Transfer. Plant and Cell Physiology, (2021) , DOI: https://doi.org/10.1093/pcp/pcab072
12. Song D.F., Jiao H.Z. and Liu Z.F.*. Phospholipid translocation captured in a bifunctional membrane protein MprF. Nat. Commun. 2:2927. (2021). (To read the article, please follow the Springer Nature SharedIt link at https://rdcu.be/ckO12)
13. Sheng X., Watanabe A., Li A.J., Kim E., Song C.H., Murata K., Song D.F., Minagawa J.* and Liu Z.F.*. Structural insight into light harvesting for photosystem II in green algae. Nat. Plants 5:1320-1330. (2019). (To read the article, please follow the Springer Nature SharedIt link at https://rdcu.be/bYFbR)
14. Jiao H.Z.,Yin Y and Liu Z.F.*. Structures of the Mitochondrial CDP-DAG Synthase Tam41 Suggest a Potential Lipid Substrate Pathway from Membrane to the Active Site. Structure 27, 1258–1269(2019).
15. Liu X. Y., Chai J.C., Ou X. M., Li M. and Liu Z.F.*. Structural Insights into Substrate Selectivity, Catalytic Mechanism, and Redox Regulation of Rice Photosystem II Core Phosphatase. Molecular Plant, 1-13, DOI: https://doi.org/10.1016/j.molp.2018.11.006. (2018).
16. Pan X.W., Ma J., Su X.D., Cao P., Chang W. R., Liu Z. F., Zhang X.Z.* and Li M.*. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science, 360:1109-1113 (2018).
17. Sheng X., Liu X. Y., Cao P., Li M and Liu Z.F.*. Structural roles of lipid molecules in the assembly of plant PSII-LHCII supercomplex. Biophys. Rep. 4, 189-203 (2018).
18. Li A.J. and Liu Z.F.*. Supramolecular structural basis of the light-harvesting process in plants. Prog. Biochem. Biophys.(生物化学与生物物理进展) 45, 935-946 (2018).
19. Cao P. ,Su X.D., Pan X.W., Liu Z. F., Chang W. R., and Li M.*. Structure, assembly and energy transfer of plant photosystem II supercomplex. Biochim. Biophys. Acta Bioenergeticcs 1859, 633-644 (2018).
20. Su X.D., Ma J.,Wei X. P., Cao P., Zhu D.J., Chang W. R., Liu Z. F.*, Zhang X.Z.* and Li M.*. Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science, 357: 815-820 (2017).
21. Ou X. M., Guo J. L., Wang L. F., Yang H. T., Liu X. Y., Sun J. Y. and Liu Z. F.*. Ion- and water-binding sites inside an occluded hourglass pore of a trimeric intracellular cation (TRIC) channel. BMC Biology 15,31 (2017).
22. Yang H.T., Hu M.H., Guo J.L., Ou X.M., Cai T.X. and Liu Z. F.*. Pore architecture of TRIC channels and insights into their gating mechanism. Nature, 538, 537-541 (2016).
23. Wei X. P., Su X.D.,Cao P., Liu X. Y., Chang W. R., Li M.*, Zhang X.Z.* and Liu Z. F.*. Structure of spinach photosystem II- LHCII supercomplex at 3.2 Å resolution. Nature, 534, 69-74 (2016).
24. Li J., Guo J. L., Ou X. M., Zhang M. F., Li Y.Z. and Liu Z. F.*. Mechanical coupling of the multiple structural elements of the large-conductance mechanosensitive channel during expansion. Proc. Natl. Acad. Sci. USA, 112: 10726-10731. (2015).
25. Wei X. P., Guo J. T., Li M.and Liu Z. F.*. Structural Mechanism Underlying the Specific Recognition between the Arabidopsis State-Transition Phosphatase TAP38/PPH1 and Phosphorylated Light-Harvesting Complex Protein Lhcb1. Plant Cell, 27: 1113-1127. (2015), DOI: 10.1105/tpc.15.00102
26. Liu X. Y., Yan Y., Wu J. J. and Liu Z. F.*. Structure and mechanism of an intramembrane liponucleotide synthetase central for phospholipid biosynthesis. Nat. Commun. 5:4244. (2014).
27. Pan X. W., Liu Z. F.*, Li M. & Chang W. R.*. Architecture and function of plant light-harvesting complexes II. Curr. Opin. Struct. Biol. 23:515-525. (* Corresponding authors) (2013).
28. Guo J. T., Wei X. P., Li M., Pan X. W., Chang W. R.* & Liu Z. F.*. Structure of the catalytic domain of a state transition kinase homolog from Micromonas algae. Protein & Cell 4, 607-619. (2013).
29. Liu Z.F.,Walton T.A.,Rees D.C. A reported archaeal mechanosensitive channel is a structural homolog of MarR-like transcriptional regulators. Protein Science 19,808-814. (2010).
30. Liu Z.F.,Gandhi C.S.,Rees D.C. Structure of a tetrameric MscL in an expanded intermediate state. Nature 461,120-124. (2009).
31. Liu Z.F.,Chang W.R. Structure of the light-harvesting complex II. In: Photosynthetic Protein Complexes. Wiley-VCH (Book chapter) Fromme P. ed., 217-242. (2008).
32. Liu Z.F.,Chang W.R. Crystallization Methods of Membrane Proteins: Practical Aspects of Crystallizing Plant Light-Harvesting Complexes. In: Biophysical Techniques in Photosynthesis II. Spinger (Book chapter)Aartsma T. J. and Matysik J. eds. 26,77-96. (2008).
33. Yan H.C.,Zhang P.F.,Wang C.,Liu Z.F.,Chang W.R. Two lutein molecules in LHCII have different conformations and functions: Insights into the molecular mechanism of thermal dissipation in plants. Biochem. Biophys. Res. Commun. 355,457-463. (2007).
34. Pascal A.A.,Liu Z.F.,Broess K.,Oort B.V.,Amerongen H.V.,Wang C.,Horton P.,Robert B.,Chang W.R.,Ruban A. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436,134-137. (2005).
35. Liu Z.F.,Yan H.C.,Wang K.B.,Kuang T.Y.,Zhang J.P.,Gui L.L.,An X.M.,Chang W.R. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428,287-292. (2004).
(From Zhenfeng Liu, October 30, 2025)

