IBP Scientists Have Developed A New Fluorescence Labeling Strategy to Allow Application of Single Molecule FRET to Dimeric Proteins
Single molecule fluorescence resonance energy transfer (smFRET) is a powerful method to reveal the conformational heterogeneity and dynamics of biomacromolecules, which is crucial for understanding the relationship between structure and function. However, the most widely applied cysteine-maleimide reaction has limitations for the fluorescence labeling of homodimeric proteins. In previous studies, methods such as subunit exchange or truncation were usually used to obtain heterodimers or monomers for FRET labeling, but these methods have obvious limitations.
The study entitled A co-expression strategy to achieve labeling of individual subunits within a dimeric protein for single molecule analysis was published as the inside front cover article in ChemComm on July 21, 2017. In this study, based on a co-expression system, Prof. Sarah Perrett and Dr. WU Si from IBP, CAS, present a generic method for site-specific incorporation of a single donor-acceptor dye pair into any desired position in a dimeric protein. The yeast prion protein Ure2 forms a highly stable homodimer and does not undergo subunit exchange on a measurable timescale. We therefore applied this co-expression strategy to label Ure2 with sets of FRET dye pairs introduced at different sites within the dimer. By measuring the distances between the dye pairs, single molecule FRET provides insight into the conformation of the intrinsically disordered prion domain of Ure2 in the context of its globular C-terminal domain. The results reveal the spatial position and the collapsed nature of the prion domain, providing clues to the inter-domain relationship. This labeling method provides a new approach to probe the structure or conformation of IDPs that are not monomeric, and has potential applications in the smFRET study of other dimeric proteins that have important biological functions.
Prof. Sarah Perrett and Dr. WU Si are the corresponding authors. LOU Fei, a PhD student in the group of Prof. Sarah Perrett is the first author. The work was funded by the National Natural Science Foundation of China and the 973 Program of the Ministry of Science and Technology.
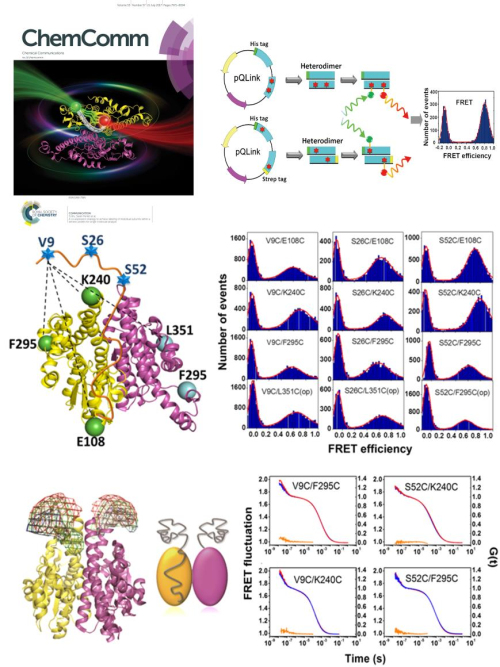
Legend: The co-expression strategy used to obtain labeled heterodimers of Ure2, in order to study the conformation and dynamics of the Ure2 prion domain by single molecule FRET. (Image by IBP)
CONTACT:
Sarah Perrett, Wu Si
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64889870
Email: sperrett@ibp.ac.cn
wusi@moon.ibp.ac.cn

