What Is the Intrinsic Dynamic Regulation of the Nuclear Membrane Protein SUN2?
CAS researchers at the Institute of Biophysics have developed a model for regulation of the nuclear membrane protein SUN2. Their work, entitled Coiled-coil Domains of SUN Proteins as Intrinsic Dynamic Regulators, was published online by the journal Structure on December 10.
The research was conducted by Ms. NIE Si and Dr. KE Huimin under the direction of Prof. FENG Wei of the Institute of Biophysics of the Chinese Academy of Sciences.
In eukaryotic cells, the nuclear envelope (NE) is a double-membrane structure that serves as a physical barrier to partition the nuclear and cytoplasmic environments. The NE is composed of the inner and outer nuclear membranes (ONM and INM, respectively) and more than 60 putative integral membrane proteins have been found to reside in either the INM or the ONM to secure the integrity of these structures.
The evolutionarily conserved SUN and KASH proteins from the ONM and INM, respectively, interact with each other in the perinuclear space to form SUN-KASH complexes that span the NE. SUN proteins can bind to the nuclear lamina in the nucleus, while KASH proteins associate with the cytoskeleton in the cytoplasm.
Thus, SUN-KASH complexes in the NE establish the physical linkage between the cytoskeleton and nuclear lamina, which is instrumental for mechanical force transmission from the cytoplasm to the nuclear interior and is essential for cellular processes such as nuclear positioning and migration, centrosome-nucleus anchorage, and chromosome dynamics.
The SUN domain of SUN proteins and the KASH domain of KASH proteins are the core components of the SUN-KASH complex. Besides the SUN domain, SUN proteins contain at least one coiled-coil domain preceding the SUN domain. Recently, it has been shown that the coiled-coil domains of SUN proteins can directly modulate SUN domain activity and regulate the subsequent formation of SUN-KASH complexes, although the underlying molecular mechanism is poorly understood.
The CAS researchers performed structural studies of the two coiled-coil domains (CC1 and CC2) of SUN2 (using mouse-sourced SUN protein). They found that CC1 and CC2 exhibit distinct oligomeric states, i.e., CC1 forms a trimer but CC2 is a monomer. The structure of the CC2-SUN monomer revealed that CC2 unexpectedly folds into a three-helix bundle that interacts with the SUN domain and locks it in an inactive conformation, while the structure of the CC1 trimer demonstrated that CC1 is an imperfect coiled-coil for the trimerization and activation of the SUN domain.
They further demonstrated that modulations of CC1 and CC2 can dictate SUN-KASH complex formation and suggested that the two coiled-coil domains of SUN2 act as the intrinsic dynamic regulators for controlling the SUN domain activity. By combining data from structural, biochemical and cellular studies, they finally provided an attractive working model illustrating how the coiled-coil domains dynamically regulate the SUN domain of SUN2.
This research was supported by grants from the National Major Basic Research Program of China and the National Natural Science Foundation of China.
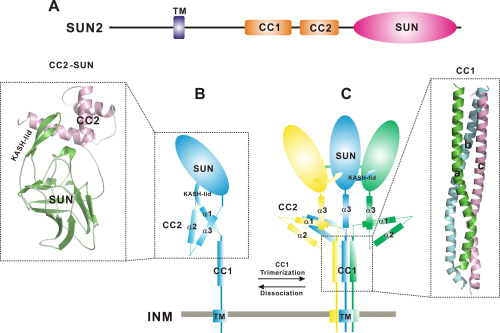
Figure 1: A schematic working model for the coiled-coil-mediated regulation of SUN2 (Image by IBP)
Contact:
FENG Wei
Institute of Biophysics, Chinese Academy of Sciences
http://www.ibp.cas.cn/ktzz/ktzz_AG/201305/t20130506_3833232.html
Email: wfeng@ibp.ac.cn

