Structure of plant photosynthetic water-splitting machinery deciphers the supramolecular bases for light-harvesting process
Photosynthesis, one of the most important biochemical and biophysical processes on earth, provides food and energy for nearly all living organisms (including human beings) in biosphere. Studying the structures and mechanisms of various machineries operating in photosynthesis will potentially offer potential illuminating solutions for solving the more-and-more pressing problems concerning energy, food and environment. These problems pose major restraints on the sustainable development of human society. The primary reactions in plant photosynthesis starts from a water-splitting machinery named photosystem II (PSII). Plant PSII contains two major modules named termed light-harvesting system (known as LHCII) and the core complex system. The core complex harbors a photosynthetic reaction center and an oxygen-evolving center capable of splitting water molecules under ambient temperature. The reactions in the core complex are powered by the light-harvesting system which absorbs light energy and transmit it to the core complex. The detailed architecture of plant PSII remains elusive for decades and a high-resolution structure of this gigantic enormously large supramolecular complex is highly anticipated in the fields of structural biology and photosynthesis research.
Recently, LIU Zhenfeng’s group, ZHANG Xinzheng’s group and CHANG Wenrui-LI Mei group from the Institute of Biophysics at CAS collaborate and have solved the structure of spinach PSII-LHCII supercomplex at 3.2 ? resolution through single-particle cryo-electron microscopy (cryo-EM). The work has been published as an "Article" in Nature on May 18, 2016 (Link to the article: http://www.nature.com/nature/journal/vaop/ncurrent/full/nature18020.html). WEI Xuepeng (Ph.D graduate student from LIU Zhenfeng’s lab) and SU Xiaodong (research assistant from CHANG Wenrui-LI Mei’s lab) are two leading authors of this work with equal contributions.
The spinach PSII-LHCII supercomplex has a total molecular mass at 1.1 megadalton (1 megdalton = 1*106 dalton) and forms a homodimer with two identical units. Each unit contains over 25 protein subunits, 105 chlorophylls, 28 carotenoids and numerous other cofactors. The sophisticated overall structural features and the arrangement of each individual subunits within the supercomplex have been revealed in this study. Three different types of LHCII, namely a major LHCII trimer and two minor LHCII named CP29 and CP26, associate at the periphery of each core complex of spinach PSII. The assembling between these three types of LHCII and the core complex is mediated by three small subunits and their binding sites have been located within the supercomplex. The result explains the essential roles of these small subunits in stabilizing the PSII-LHCII supercomplex as discovered in the previous functional studies.
The details concerning the energy-transfer pathways between LHCII and the core complex in the PSII-LHCII supercomplex have were enigmatic before this workremained elusive for decades. The information is vital for understanding the light-harvesting process occurring in plants. By analyzing the pigment networks within the spinach PSII-LHCII supercomplex, the specific pathways between LHCII, CP29, CP26 and the core antenna complexes CP43/CP47 have been defined and described in great details. Moreover, the potential energy-quenching sites (forming under high-light conditions) functioning in a regulatory process (known as photoprotection) have been located within the supercomplex. These results are fundamental and will help to advance further researches on the kinetics of energy transfer and photoprotection mechanism within PSII-LHCII supercomplex.
The research was funded by National 973 project from the Ministry of Science and Technology of People’s Republic of China, the Strategic Priority Research Program of CAS and National Natural Science Foundation of China. Cryo-EM data collection was carried out at the Center for Biological Imaging, Core Facilities for Protein Science at IBP, CAS, and at the National Center for Protein Science Shanghai (NCPSS), Shanghai Institutes for Biological Sciences/Shanghai Science Research Center, CAS.
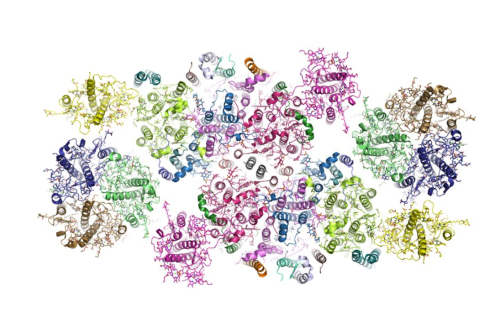
Figure: The structure of spinach PSII-LHCII supercomplex (Image by IBP)
Contact
LIU Zhenfeng
National Laboratory of Biomacromolecules, IBP,CAS
Tel:010-64881481(off.), 010-64889535 (lab)
E-mail:liuzf@sun5.ibp.ac.cn
ZHANG Xinzheng
National Laboratory of Biomacromolecules, IBP,CAS
E-mail: xzzhang@ibp.ac.cn
LI Mei
National Laboratory of Biomacromolecules, IBP,CAS
E-mail:meili@moon.ibp.ac.cn

