FUS Interacts with HSP60 to Promote Mitochondrial Damage
FUS (fused in sarcoma/translocated in liposarcoma,FUS/TLS), a DNA/RNA binding protein, is predominantly localized in nucleus, but is also known to shuttle between the nucleus and the cytoplasm. FUS protein has been associated with multiple steps in RNA metabolism, including RNA transcription, RNA splicing and microRNA processing. FUS-proteinopathies, a group of heterogeneous neurodegenerative disorders including Amyotrophic Lateral Sclerosis with FUS pathology (ALS-FUS) and Frontotemporal Lobar Degeneration with FUS pathology (FTLD-FUS), are characterized by the formation of inclusion bodies containing the nuclear protein FUS in the affected patients. So far, more than 50 mutations in the FUS gene have been found in patients with FUS-proteinopathy. However, the underlying molecular and cellular mechanisms of FUS in these diseases remain unclear.
In a new study published online September 3rd in PLOS Genetics, entitled FUS Interacts with HSP60 to Promote Mitochondrial Damage, Prof. WU Ying’s Group from Institute of Biophysics made new progress in the molecular and cellular mechanisms of FUS in heterogeneous neurodegenerative diseases, which was supported by Prof. XU Qi from Peking Union Medical College (PUMC) and Prof. DU Sidan from Nanjing University. The FTLD-FUS patient samples were contributed by Prof. Eileen H. Bigio and Prof. Marsel Mesulam from Northwestern University Feinberg School of Medicine. Previous work from Prof. WU’s lab has established a drosophila model of FUS-proteinopathy, which could recapitulate major clinical and pathological features of these devastating diseases (Chen et al., 2011).
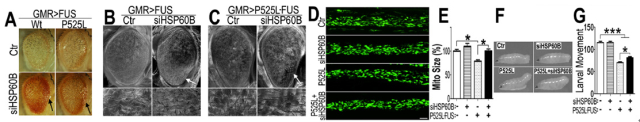
Based on the animal model, this study further demonstrated that over-expression of FUS caused mitochondrial fragmentation and damage. By using genetic screening, FUS was found to interact with mitochondrial chaperon HSP60 and RNAi-mediated down-regulation of the HSP60 homolog partially rescued the neurodegenerative phenotypes in FUS transgenic flies (Figure 1). Immuno-electron microscopy (IEM) and biochemical studies showed HSP60 mediated FUS localization to the mitochondria.
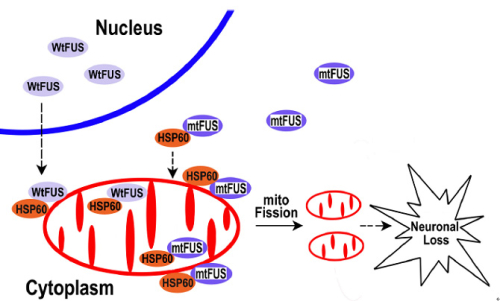
The findings of the present study suggested that under pathological conditions or FUS mutations could increase FUS localization to the mitochondria, leading to mitochondrial damage and finally neuronal cell death (Figure 2). Mitochondrial impairment may be an early event in FUS-proteinopathies and represent a potential therapeutic target for treating these fatal diseases.
Figure 1. Down-regulating fly HSP60B expression partially rescues neurodegeneration phenotypes in FUS transgenic flies.
Figure 2. A working model for FUS-induced neurotoxicity.