The mechanical coupling mechanism of a mechanical force-sensing channel protein discovered by scientists from the Institute of Biophysics at CAS
Being able to sense and respond to mechanical force stimuli is fundamental to animals, plants and microbes (such as bacteria and yeast). The process of mechanical-force signal transduction is mediated by a class of channel proteins named mechanosensitive channels. They are located on the cell membrane and sensitive to mechanical forces, such as the stretching force induced by a pressure applied on the membrane. The large-conductance mechanosensitive channel (MscL) helps the microbial cells to sense the membrane tension, regulate the osmotic pressure balance between the intracellular and extracellular environments, and thus are vital for the bacteria and other microbes to survive in the constantly changing environments. Recent studies demonstrated that MscL can provide the portal for antibiotics to enter bacterial cells and may serve as a potential target for the antibiotics to act on. Moreover, it can be modified into a light-activated channel for the release of liposome-encapsulated drugs. For basic research, MscL is believed to be an ideal model system for studying the mechanism of mechanical force transduction across the membrane. Toward elucidating the principle underlying the operation of MscL like a nanoscale mechanical valve, it emerges as an indispensible and challenging task for researchers to characterize the different conformational states of MscL during its gating process through structural biology and other approaches.
In a recent study, LI Jie and colleagues (led by Dr. Zhenfeng Liu at the Institute of Biophysics of Chinese Academy of Sciences) solved the crystal structures of a MscL homolog from a methane-producing microbe (Methanosarcina acetivorans) in the closed and expanded states, revealing snapshots of two distinct conformational states of MscL. Through an in-depth geometric analysis and comparison of these two structures, the principle underlying the conformational transition between the two states has been discovered. The study demonstrates that the membrane-spanning domain of MscL goes through a large-degree tilting motion during expansion, resembling that of an camera iris. Furthermore, the N-terminal helix part of MscL ("umbrella rib") is stretched to a horizontal position by the membrane-spanning domain ("umbrella stretcher") as if an umbrella is opened. When MscL expands, the different parts of the channel are mechanically coupled tightly and cooperate with each other so that the different subunits of the channel can synchronize their action. On methodology, they have invented a fusion combination of a pentameric riboflavin synthase (MjRS) and MaMscL to promote the crystallization of MaMscL through an extensive designing and screening process. Such a fusion channel protein can be trapped in two distinct conformational states under different detergent-solution conditions. The method not only solved the long-standing problem of studying MscL through crystallography, but may also provide an illuminating example for future structural biology researches on those homo-oligomeric eukaryotic ion channels with important biological functions.
The work is entitled " Mechanical coupling of the multiple structural elements of the large-conductance mechanosensitive channel during expansion" and published online by the PNAS journal on Aug 10, 2015. The research was funded by the National 973 Project Grants, the Strategic Priority Research Program of Chinese Academy of Sciences, 135 project and startup funding from Chinese Academy of Sciences. Dr. Jianli Guo and Xiaomin Ou from IBP, CAS and Dr. Yuezhou li and his colleague Mingfeng Zhang from Zhejiang University performed the electrophysiological studies in this research. The X-ray diffraction data were collected at the BL17U beamline of Shanghai Synchrotron Radiation Facility and BL17A beamline of the Photon Factory. Dr. Jianyuan Sun's group provided the access to their electrophysiological devices used in this work.
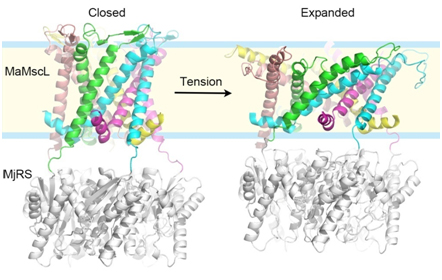
Figure legend:Three-dimensional structures of MaMscL at two different conformational states. Colorful ribbons:the closed-state (left) and expanded-state (right) MaMscL structures; Each color code (green, cyan, magenta, yellow and pink) labels an individual subunit of the channel. Silver ribbons, the MjRS protein used for facilitating the crystallization of MaMscL. (Image by LI Jie and LIU Zhenfeng)

