A new technique developed by collaboration of IBP and University of Cambridge allows researchers to identify clusters of proteins on immune cells which are key to fighting off the body’s invaders.
When our bodies are under attack from foreign organisms, such as bacteria and viruses, our immune system orchestrates a complex fight-back involving many separate parts. One important component of this response is a type of cell called the B-lymphocyte – it is this cell that is at the forefront of our defence as it identifies and attempts to neutralise invaders.
The B-lymphocyte produces a protein called the B-cell receptor on its surface. The receptor recognises and attaches itself to molecules from the invading organisms, known as antigens. This triggers the B-lymphocyte to divide and to release specialised proteins called antibodies which neutralise the antigens.
There are many aspects of this process that are still not well understood. One reason is because the B-cell receptor does not exist in isolation on the B-lymphocyte surface. Rather, it forms localised clusters together with a number of ‘molecular neighbours’. It is these local interactions that control how the lymphocytes divide and replicate and determine the strength of the antibody response. A better understanding of these interactions could ultimately lead to better control of the immune response – for example in vaccine development. However, the molecular contacts within the clusters are relatively weak, and so they are technically difficult to identify.
Now, in an international collaboration, scientists at the Chinese Academy of Sciences Institute of Biophysics (IBP), the University of Cambridge’s Department of Biochemistry and the Cambridge Centre for Proteomics have developed a technique that allows some of these molecules to be detected. It is published in 23rd May edition of the Journal of Biological Chemistry (http://www.jbc.org/content/289/21/14434.full.pdf+html). The method enables proteins in the immediate vicinity of the B-cell receptor to be chemically tagged in such a way that they can be more easily isolated. The tagged molecules can then be identified using a method called mass spectrometry.
For this initial ‘proof of principle’ experiment, the researchers looked at the B-cell receptor on the surface of a chicken B-lymphocyte and identified molecules that were hitherto not thought to be involved in regulation of the receptor. They show that these molecules combine with the receptor to activate a class of proteins called integrins that are known to play an important role in the response of B-lymphocytes to antigens. Similar molecules occur on the human B-lymphocyte surface, and drugs active against integrins are already used to modulate the immune response. So a long-term implication of this work may be to identify new therapeutic targets for immune regulation.
The experiments were performed primarily by Xue-Wen Li in Beijing and Dr Jo Rees in Cambridge. The work was supervised by Professor Sarah Perrett from the Institute of Biophysics in Beijing, Dr Tony Jackson from the Department of Biochemistry, Cambridge and Professor Kathryn Lilley in the Cambridge Centre for Proteomics. Assistance in mass spectrometry was also provided by the group of Professor Fuquan Yang in the IBP MS Center, Beijing. The experiments on integrin activation were performed with Professor Richard Farndale in the Department of Biochemistry Cambridge.
Professor Perrett said: “In applying this technique, we have addressed a particularly challenging issue: how do we identify weak and transient, but potentially important, interactions between membrane proteins, which are notoriously difficult to work with?”Dr Tony Jackson said: “There are many problems in cell-biology where we would like to identify proteins that group together on the cell surface, and our method could also be applied in these cases. It should therefore be of interest to a wide group of researchers in both the academic and industrial biomedical communities.”
Funding for the research included the Chinese Ministry of Science and Technology 973 Program (2012CB911000, 2013CB910700), the National Natural Science Foundation of China (31110103914, 31070656, 31000342, 31270794) and a Chinese Academy of Sciences Visiting Professorship for Senior International Scientists (2010T1S11) which enabled Dr Tony Jackson to visit the IBP.
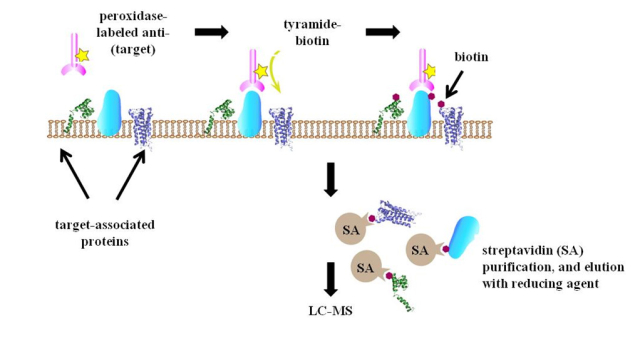
Legend: Principle of SPPLAT developed in our research. The antibody-directed targeting of HRP to a surface protein of interest, followed by brief labeling with biotin-tyramide enables proteins in the immediate vicinity of the target to be biotinylated. These are isolated by incubation of the cell lysate with streptavidin-agarose (SA), and elution with reducing agent.
Note: link to the Cambridge University press release: http://www.cam.ac.uk/research/news/neighbourhood-watch-new-technique-helps-identify-proteins-involved-in-immune-response

