The snapshots ofa key step in the photosynthetic state transitions captured by scientists from the Institute of Biophysics at CAS
The natural environments are full of changes. For instance, the light quality and intensity fluctuate constantly during the day. The algae and land plants are able to respond and counteract the imbalance caused by the fluctuation of light conditions. At molecular level, the adaptation is achieved through a series of regulatory mechanisms, including the processes well known as state transitions. During state transitions, plants respond to the changes of light quality and balance the distribution of excitation energy between the two photosystems (photosystem I [PSI] and photosystem II [PSII]) that catalyze the conversion of light energy during photosynthesis. The state transitions represent a fundamental acclimation mechanism for plants to thrive in the constantly changing natural environment. The state-transition processes are dependent on reversible phosphorylation and dephosphorylation of the major light-harvesting complex LHCII. A protein phosphatase named TAP38/PPH1 is essential for the dephosphorylation of LHCII and the transition from state 2 to 1. It was originally identified through molecular genetics by two different groups (Leister group in German and Goldschmidt-Clermont group in Switzerland) independently in 2010. Nevertheless, it remains enigmatic about how TAP38/PPH1 and phosphorylated LHCII recognize each other during state transitions.
In a recent work published by the Plant Cell journal, Xuepeng Wei (first author) and colleagues(led by Dr. Zhenfeng Liu at the Institute of Biophysics at CAS, webpage: http://english.ibp.cas.cn/ibp_pi/L/201312/t20131202_113564.html) demonstrate that the state transition phosphatase TAP38/PPH1 can act directly on the phosphorylated LHCII and efficiently catalyzes dephosphorylation of LHCII. Subsequently, they solved the crystal structures of Arabidopsis state transition phosphatase TAP38/PPH1 itself and TAP38/PPH1 in complex with N-terminal phosphorylated peptide of Lhcb1 (one of the three major proteins of LHCII). By comparing and analyzing the two structures, they have discovered that TAP38/PPH1 contains two specialized motifs involved in recognizing the substrate and forming direct interactions with pLhcb1 phosphopeptide. Furthermore, the crystal structure of TAP38/PPH1 in complex with the substrater presents the first crystal structure of type-PP2C(protein phosphatase 2C) phosphatase in complex with phosphorylated peptide, and thus provides important details for an in-depth understanding on the catalytic process of PP2C family.
This work was entitled "Structural mechanism underlying the specific recognition between the Arabidopsis state-transition phosphatase TAP38/PPH1 and phosphorylated light-harvesting complex Protein Lhcb1" and published online by the Plant Cell journal on April 17, 2015. The research was funded by the National 973 Project Grants 2011CBA00903 and the Strategic Priority Research Program of CAS (XDB08020302). Drs. Mei Li and Jiangtao Guo from Prof. Wenrui Chang's group also participatein this research. The X-ray diffraction data was collected at the BL17U beamline of Shanghai Synchrotron Radiation Facility and NW12A beamline of the Photon Factory.
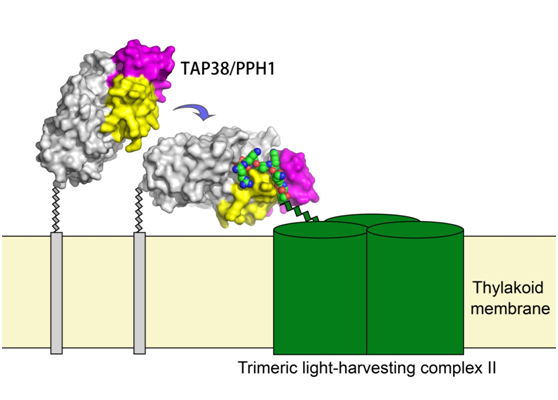
Figure legend. The interaction between the state-transition phosphatase TAP38/PPH1 and the light-harvesting complex LHCII. Color codes: green cylinders, LHCII; green-blue-red tricolor sphere model, the N-terminal phosphopeptide of Lhcb1; blue arrow, the switch of TAP38/PPH1 from the substrate-free state to substrate-loaded state.
CONTACT:
Prof. Zhenfeng Liu
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China.
E-mail: liuzf@sun5.ibp.ac.cn

