Crystal structure makes light-harvesting complex clearer
CP29 is one of the minor light-harvesting complexes of higher plant photosystem II (PSII) embedded in the thylakoid membrane. It is located between the major light-harvesting complex LHCII and the core complex. CP29 is necessary for PSII organization and a key component for the stability of the PSII–LHCII supercomplex. Besides the efficient light-harvesting and energy transfer functions, CP29 plays a bridging-type role in transferring the excitation energy from the outmost antenna LHCII to the reaction center. In recent years, there has been increasing evidence that CP29 is important for photoprotection, through which excess energy can be dissipated safely and plants can be protected from photodamage.
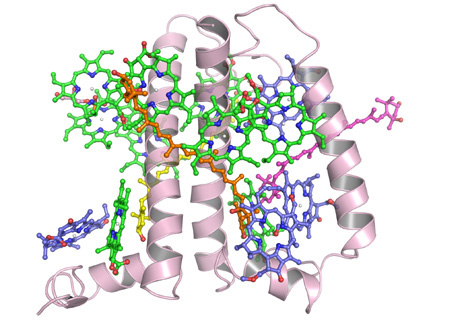
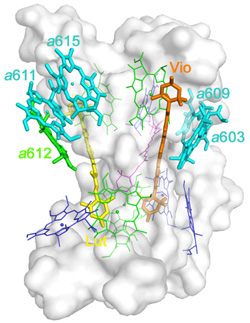
Recently, Professor Wenrui Chang and his colleagues at the Institute of Biophysics, Chinese Academy of Sciences had successfully determined the crystal structure of the minor light-harvesting complex CP29 from Spinacia oleracia at 2.8 angstrom resolution. The work was online published in Nature Structural & Molecular Biology(February 6, 2011).The crystal structure revealed that the apoprotein of CP29 has three transmembrane α-helices, and two short amphipathic helices located on the lumenal surface. Each CP29 monomer contains 13 chlorophyll (Chl) and 3 carotenoid molecules, which differs considerably from the previously widely used predicted CP29 model. The 13 chlorophyll-binding sites are assigned as eight Chl a sites, four Chl b and one putative mixed site occupied by both Chls a and b. In the crystal structure, five chlorophylls are newly discovered, compared with the previously predicted CP29 model. A special pigment pair, which share one central ligand, forming a sandwich structure is discovered. This particular mode of coordination is the only example seen in pigment–protein complexes of the photosystem so far. Based on the present X-ray structure, an integrated pigment network in CP29 is constructed. Two special clusters of pigment molecules, namely a615–a611–a612–Lut and Vio(Zea)–a603–a609, have been identified at the surface of CP29 and might function as potential energy-quenching centers and as the exit or entrance in energy-transfer pathways.
The three-dimensional struc-ture of CP29 is essential for gaining an in-depth insight into the mechanisms of light harvesting, energy transfer and the photoprotection functions of CP29. It also provides an opportunity for us to obtain an improved understanding on the minor peripheral antenna of PSII.
Professor Chang’s group had been focusing on this study for more than five years. After the significant breakthrough in crystal structure determination of LHCII, this is another important achievement that the group had made in solving the crystal structure of photosynthesis membrane protein CP29.
 |
 |
| The overall structure of CP29 monomer |
The pigment network in CP29 monomer |

