Progress in artificial design of light driven carbon dioxide reductase by WANG Jiangyun’s group
In Nov 5th, 2018, Nature Chemistry published prof Jiangyun Wang’s researching article ”A genetically encoded photosensitizer protein facilitates the rational design of a miniature photocatalytic CO2 reducing enzyme”. This paper reported a rational designed artificial protein which successfully mimicked the CO2 photoreduction activity of natural photosystems.
Searching methods to control increasing atmosphere CO2 concentration has become a important task in chemistry research areas. Natural photosynthesis systems have attracted lots of attention, because of their advantages including environment friendly, self-assemble ability and highly efficient photo-induced charge separation efficiency. However, when be compared to small molecule CO2 reduction catalyst, the CO2 reduction activity of natural photosynthesis system is relatively lower. Additionally, a natural photosystem is usually composed by a set of protein subunits and cofactors, which hindered its application in both laboratory and industry. In order to combined the advantages of natural photosystem and small molecule catalysts, Prof. Jiangyun Wang’s group has sped years in designing genetic encoded artificial protein to reduce CO2. This protein catalyst would provide new sights in designing highly efficient CO2 reduction catalyst. It would also facilitate designing artificial organisms with unnatural photocatalysis activities.
The key problem in designing a fluorescent protein based CO2 reduction enzyme is to obtain an excited protein state with long lifetime and low reduction potential. Our previous studies have proved that fluorescent proteins with only 27 kD molecule weight could be transformed into photocatalysts. Upon light irradiation, the exited chromophore of fluorescent proteins could transfer an electron to a receptor located on the outside surface of the protein beta-barrel. Meanwhile, the chromophore could be rational designed by replacing its natural tyrosine residue with a genetic encoded unnatural amino acid. Thus, the photochemistry features of the chromophore, including absorption spectrum, life time and radical reductive potential, could be rational attuned with high accuracy (Angew. Chem. Intl. Ed. 2012, 51, 10261-5;Angew. Chem. Intl. Ed. 2013, 52, 4805-9;J. Am. Chem. Soc. 2014, 136 , 13094-7;J. Am. Chem. Soc., 2015,137,7270-3).
In this article, researchers engineered the fluorescent protein chromophore by incorporating an unnatural amino acid (BpA) containing a benzophenone side chain. Benzophenone is a commonly used photosensitizer in organic photocatalysis. Excited benzophenone molecule would quickly turn to a triplet state with a longer lifetime. Then it would react with a sacrificial reductant and generate a highly active radical. That feature of benzophenone was conserved when be incorporated into fluorescent proteins. Time resolved absorption spectrum proved that engineered fluorescent protein (photosynthesis protein, PSP) would turn into a triplet sate with close to 100 percent efficiency upon light irradiation. With the presence of sacrificial reductant such as ascorbate acid, the triplet sate would then turn into a radical state with more than 10 minutes lifetime. Additionally, electrochemistry analysis suggested the redox potential of radical state is close to -1.5 V, which is much lower than CO2 reduction potential requirements and natural reductants.
After that, we construct a holo CO2 photo-reductase by labeling the photosynthesis protein with an organic metal complex molecule nickel-terpyridine. the hybrid protein catalyzed CO2 to CO photoreduction conversion with more than 100 total turnover numbers and more than 2.6% quantum yield, which is higher than most reported CO2 photoreduction catalysts.
This study was accomplished by Prof. Jiangyun Wang’s group of institutes of biophysics. Chinese academy of sciences. This study was supported from the National Key Research and Development Program of China under Award 2017YFA0503704, 2016YFA0501502, the National Science Foundation of China under award 21750003, 91527302, U1632133, 31628004, 21473237, 31628004 and Key Research Program of Frontier Sciences, CAS, Grant No. QYZDB-SSW-SMC032 and 15PTCY0020,ZVSM201811092.
Article link: https://www.nature.com/articles/s41557-018-0150-4
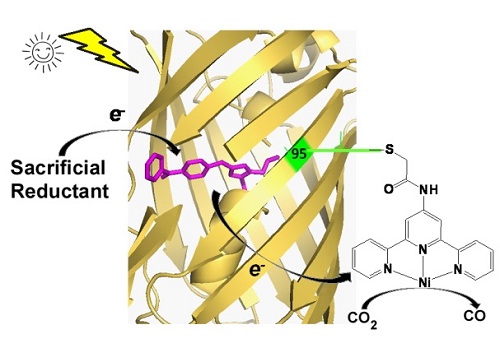
Schematic diagram of proposed catalytic mechanism of PSP2T
Contact: Jiangyun Wang
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101,China
Phone: 86-10-64852570
Email: jwang@ibp.ac.cn

