Structure of an ancient prokaryotic photosynthetic core complex
Photosynthesis is the primary solar energy transformation process that powers life on Earth. In order to efficiently capture solar radiation, diverse photosynthetic apparatuses have evolved in different types of photosynthetic organisms. Two photosystems work together in tandem in alga and higher plants, whereas in cyanobacteria there are supramolecularmega-complexes found. Anoxygenic photosynthetic prokaryotes are diverse groups of bacteria thriving worldwide since the early history of the planet. In most species of photosynthetic prokaryotes, light energy is initially absorbed by the peripheral light-harvesting (LH) antenna and then transferred via the inner LH to the reaction center (RC), where the primary reaction of photosynthesis occurs. The primary separated electron is transferred within the RC to a quinone. The fully reduced quinol is then exchanged with an oxidized quinone from the membrane pool and passes its electrons to the next redox component in the cyclic electron transfer pathway, during which a transmembrane proton gradient is established for the subsequent production of ATP.
Photosynthetic members of phylum Chloroflexi, the filamentous anoxygenic phototrophs (FAPs) can survive from the environment with weak light, and have a photosynthetic system with efficiently energy and electrons transfer and high capacity for self-protection. Biochemical analysis indicated FAPs are phylogenetically distant from other anoxygenic photosynthetic bacteria and form the deepest branch of bacteria. This type of organism acquired a “chimeric” photosynthetic system during evolution, with their pheophytin/quinone reaction center resembling that in purple bacteria, while the light-harvesting apparatus is similar to green sulfur bacteria. Therefore, FAPs have received considerable attention as an important group when exploring the early evolution and diversity of photosynthesis and when developing the alternative energy.
In the paper titled ”Cryo-EM structure of the RC-LH core complex from an early branching photosynthetic prokaryote” published by Nature Communications on April 19, 2018, Prof. SUN Fei from Institute of Biophysics, Chinese Academy of Sciences and Prof. XU Xiaolingfrom Hangzhou Normal University, reported the high-resolution structure of the photosynthetic core complex, RC-LH, from Roseiflexuscastenholzii. This study indicated the molecular basis of how the energy is absorbed, transferred and transformed efficiently.
In this work, the authors used the cryo electron microscopy single particle analysis to determinethe structure of photosynthetic core complex (RC-LH) at 4.1 ? resolution. The RC-LH complex has a tetra-heme cytochrome c bound RC encompassed by an elliptical LH ring that is assembled from 15 LHαβ subunits. An N-terminal transmembrane helix of cytochrome c inserts into the LH ring, not only yielding a tightly bound cytochrome c for rapid electron transfer but also opening a slit in the LH ring, which is further flanked by a transmembrane helix from a newly discovered subunit X. These structural features suggest an unusual quinone exchange model of prokaryotic photosynthetic machinery.
Associate Prof. XIN Yueyong and Dr. SHI Yang, NIU Tongxin from SUN’s group are the co-first author in this paper. All the cryo electron microscopy work was performed in Center for Biological Imaging, Institute of Biophysics, Chinese Academy of Sciences with the contributions from Dr. HUANG Xiaojun and Dr. DING Wei. This work was supported by grants from the Strategic Priority Research Program of Chinese Academy of Sciences, the National Basic Research Program (973 Program) of Ministry of Science and Technology of China, and the grants from National Natural Science Foundation of China and Zhejiang Provincial Natural Science Foundation of China.
For full text, please see: https://www.nature.com/articles/s41467-018-03881-x
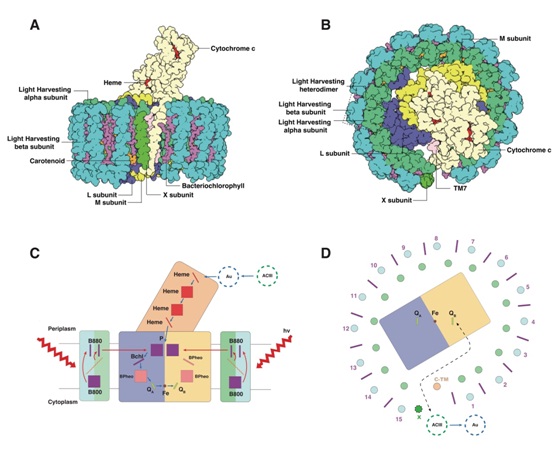
The overall architecture of RC-LH and the scheme ofthe possible way of energy and electron transfer in RC-LH.
Contact: SUN Fei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64888422
Email: feisun(AT)ibp.ac.cn

