Research progress of a novel aromatic prenyltransferase for its unprecedented enzymatic promiscuity
On December 12, 2016, Natural chemical biology reported a new research progress entitled Molecular Insights Into The Enzyme Promiscuity Of An Aromatic Prenyltransferase by Chinese Academy of Medical Sciences and Peking Union Medical College and Institute of Biophysics, Chinese Academy of Sciences. They reported a new type of aromatic prenyltransferase (AtaPT) that is cloned from microorganism with unprecedented substrate promiscuity, and discovered the relevant molecular mechanism.
Traditionally, enzymes are considered to be specific towards their substrates; however, many enzymes show substrate promiscuity, which is different from natural substrates. The promiscuity of enzymes has draw people’s attraction, but the molecular mechanism is still unclear.
The substitution reactions of prenyl moieties on natural compounds give rise to kinds of products with variety of structural types and bioactivities, which are important source for new drug discovery. The introduction of prenyl moieties into natural molecules enriches the diversity of structural types greatly, and enhances the bioactivities by increasing the lipophilic properties. Thus, an aromatic prenyltransferase with diverse substrate is an important tool for drug discovery.
In their research, a brand new prenyltransferase gene named AtaPT was discovered, and the recombinant protein AtaPT could not only accept donors with different chain length (C5,C10,C15,C20), but also different kinds of acceptors, including genestins, cycled-L-Trp, quinoline alkaloids, xanthone, dimethyl ketone, flavonoids and so on with high efficiency and many kinds of substitution reaction (O-、C-prenylation), showing great substrate promiscuity never reported before (Fig. 1).
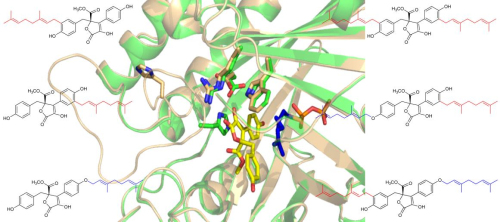
Fig. 1 Prenylation occurred at different positions of one compound by AtaPT results in different products. Comparing the apo state and substrate-bound state shows the conformational change of AtaPT induced by substrates binding. (Image by IBP)
The authors further explain the substrate promiscuity of AtaPT with systematic structural studies. Firstly, AtaPT has an enlarged hydrophobic pocket for substrates binding in comparison with other PTases reported; secondly, AtaPT has different positions for substrates binding; thirdly, AtaPT has multiple conformations to accommodate substrates binding with different size and shape. These finds also provide insights for structure-guided mutagenesis to control prenylation reactions. Their research provides an important enzyme tool to synthesis various kinds bioactive compounds for new drug discovery.
Prof. DAI Jungui from Chinese Academy of Medical Sciences and Peking Union Medical College and Prof. SUN Fei from with Institute of Biophysics, Chinese Academy of Sciences are corresponding authors of this work. Doctor CHEN Ridao and LIU Xiao from Prof. DAI Jungui’s group and Doctor GAO Bingquan from Prof. SUN Fei’s group contributes equally to this work. Prof. LOU Jizhong from Institute of Biophysics, Chinese Academy of Sciences and Prof. LI Shuming from University of Marburg also contribute to this work. This research is funded by National Natural Science Foundation、Ministry of Science and National Key Basic Research and Development Plan (973 plan) and Strategic Priority Research Program (Chinese Academy of Sciences).
Contact:

