Scientists at IBP and NIBS identified key regulators of H3K36 methyltransferase Ash1
On November 21, 2017, a study led by Dr. Bing Zhu from Institue of Biophysics (IBP), CAS and Dr. Rongwen Xi from National Institue of Biological Sciences, Beijing (NIBS) was published online in Nature Communications as an article. This work for the first time reported Mrg15 as a subunit of the Ash1 complex, a stimulator of Ash1 enzymatic activity and a critical regulator of the TrxG protein function of Ash1 in Drosophila.
Polycomb group (PcG) and Trithorax group (TrxG) proteins are two groups of factors with opposing functions and maintain the transcriptional “off” and “on” states of Hox genes, respectively. Ash1 is a TrxG protein and a SET domain containing histone methyltransferase. In 2011, Dr. Bing zhu’s group published an paper in JBC reporting the confirmation of Ash1 as an H3K36me2 methyltransferase and also revealed that H3K36 methylation can inhibit the establishment of Polycomb Repressive Complex 2 (PRC2) catalyzed H3K27 methylation, thus providing a molecular explanation for the phenomenon that TrxG antagonizes PcG mediated gene silencing. However, little was known about the enzymatic regulation of Ash1.
In this work, the reserchers perfomed affinity purification and identified Mrg15 and Nurf55 as integral subunits of Ash1 complex; in vitro biochemical assays revealed that Mrg15 greatly stimulates the enzymatic activity of Ash1; Ash1 and Mrg15 ChIP-seq experiments in Drosophila S2 cells suggested Ash1 colocalizes with Mrg15 on chromatin and Ash1 recruits Mrg15 to their common targets; H3K36me2 ChIP-seq and RNA-seq experiments suggested that Mrg15 contributes to the optimal deposition of Ash1 catalyzed H3K36me2 and transcriptional activity of target genes; To further dissect the physiological role of Mrg15 within Ash1 complex, the researchers generated a knock-in fly berrying an Ash1-R1288A mutation which can greatly attenuate the interaction between Ash1 and Mrg15. Homozygous mutant fly displays multiple homeotic transformation phenotypes and the phenotypes can be partially rescued by overexpression of Mrg15-Nurf55 fusion protein which stabilizes the interaction between Mrg15 and Ash1-R1288A. This suggested Mrg15 mediated Ash1 activation is required for the anti-Polycomb function of Ash1.
Taken together, This is an important advance that will be relevant to all who study Polycomb and Trithorax, and also shed light on further mechanism research of enzymatic regulation of histone methyltransferase.
This work was supported by the China Natural Science Foundation, Chinese Ministry of Science and Technology, the Strategic Priority
Research Program and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
Article linkage: https://www.nature.com/articles/s41467-017-01897-3
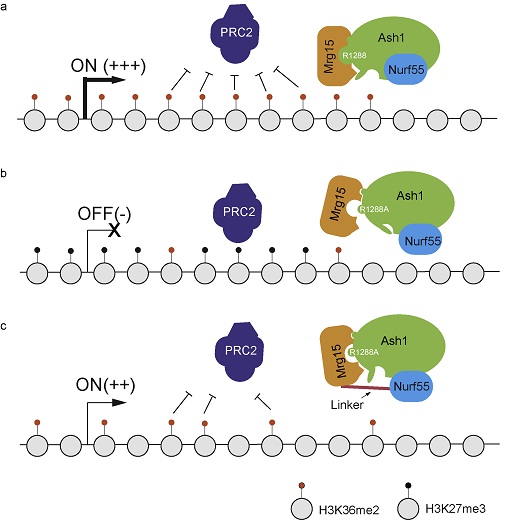
A working Model: The function of Mrg15 in Ash1 complex.

