The Structure of the Human “Marijuana Receptor”Was Resovled
A team of scientists led by LIU Zhijie at the Institute of Biophysics, CAS has determined and analyzed the high-resolution atomic structure of human cannabinoid receptor 1 (CB1), also known as the “marijuana receptor” . The new findings provide clues to understand why some drugs that interact with this receptor have had unexpectedly complex and sometimes harmful effects, while the utility of the crystal structure may provide inspiration for drug design toward refining efficacy and avoiding adverse effects.
CB1 is one of the most highly expressed G protein-coupled receptors (GPCRs) in the human central nervous system and is the target of one of the longest used herbs in human history. Previous studies discovered that CB1 is the principal target of delta9-tetrahydrocannabinol (THC), a psychoactive chemical from marijuana (Cannabis sativa) which has been used for therapeutic purposes for many centuries. CB1 is activated by endocannabinoids, and is a promising therapeutic target for pain management, inflammation, obesity, liver fibrosis and substance abuse disorders. However, CB1 has not been targeted to its full pharmaceutical potential and there is urgent need for achieving a better understanding of this receptor.
GPCRs are responsible for more than 80% of signal transmission across cell membranes and are the targets of nearly 40% of today’s marketed medicines. The detailed molecular structure of the receptor in complex with agonist or antagonist will help people understand how cellular signaling is affected by drugs, and will offer a valuable framework for designing safer and more effective medicines.
The first CB1-selective antagonist/inverse agonist, rimonabant (SR141716, AcompliaTM [Sanofi-Aventis]), received approval from the European Medical Agency (EMA) as an adjunct to diet and exercise for treating obesity. However, rimonabant and other ligands in its class were withdrawn from the EMA and were not approved in the United States due to concerns about adverse events, such as increased anxiety, depression, and suicidal ideation. Given the immense opportunities that CB1 provides as a therapeutic target, efforts have since focused on determination of the 3-D structure of CB1 with ligands to aid in the development of safer pharmacotherapies for overcoming such undesirable effects.
The Makriyannis lab at Northeastern University designed and synthesized AM6538, a strong stabilizing antagonist of CB1, specifically for structural studies.
The first author of this study, TIAN Hua, a graduate student at LIU Zhijie group, and her colleagues determined the crystal structure of CB1 in complex with AM6538 at 2.8 angstrom resolution. AM6538, synthesized by the second author Kiran Vemuri, is expected to have a long half-life and be potentially useful in the treatment of addiction disorders. Additionally, their studies found a number of structurally dissimilar CB1 ligands can be accommodated when docked into the binding domain defined by the CB1 crystal structure. This should allow a diverse set of CB1 ligands to interact with CB1 through different binding motifs and have distinct pharmacological profiles.
Funding support for these studies was provided by the Ministry of Science and Technology of China, The National Nature Science Foundation of China, National Institutes of Health, National Science Foundation, Shanghai Municipal Government, ShanghaiTech University and GPCR Consortium.
Artistic rendering of the human cannabinoid receptor CB1.
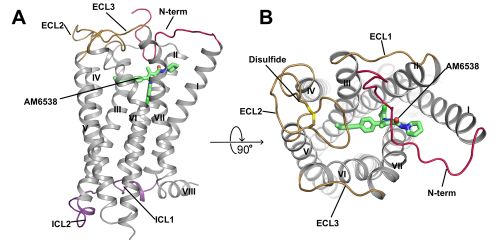
Overall Structure of CB1-AM6538 Complex. (A) Side view. (B) Top view.
Contact
LIU Zhijie
Principal Investigator (guest)
National Laboratory of Biomacromolecules, IBP
iHuman Institute, ShanghaiTech University
E-mail:liuzhj@shanghaitech.edu.cn
Tel:86-10-64888426/21-20685008

