Research Explains Molecular Mechanism of Ribosomal Back-movement During Protein Translation
New research by Prof. QIN Yan of the Institute of Biophysics (IBP) of the Chinese Academy of Sciences, in collaboration with Tsinghua University, has shown the molecular mechanism for ribosome back-movement during protein translation. The study, entitled EF4 Disengages the Peptidyl-tRNA CCA End and Facilitates Back-translocation on the 70S Ribosome, was published online on Jan 25 in Natural Structural & Molecular Biology,
Ribosome is the factory of protein translation. During translation, ribosome moves along the mRNA in the direction of 5’ to 3’, which motion is catalyzed by the translation elongation factor G (EF-G). However, the discovery of the highly conserved translation elongation factor 4 (EF4) by Dr. QIN Yan and the tRNA motions that it induces has indicated that tRNAs in the P- and E-site of the translating ribosome can move backwards, namely the ribosome can move backwards along the mRNA in the direction of 3’ to 5’.
QIN’s group has focused on ribosome translocation research since her lab was set up at IBP in 2006. In 2012 and 2014, QIN’s group twice published the molecular structure and mechanism of EF4- and EF-G-induced translation regulation in the journal Nature Structural & Molecular Biology (Figure A).
In the current study, Prof. QIN and Dr. GAO Ning of Tsinghua University combined cryo-EM and biochemical tools to characterize E. coli translating ribosomes assembled with EF4, seeking to understand the molecular mechanism by which EF4 performs its unique back-translocase function.
The researchers found that EF4 triggers tRNA back-movement by a specific region in the EF4 C-terminal domain (CTD) interfering with base-pairing between the peptidyl-tRNA 3′-CCA and the P-loop of the ribosome peptidyl transferase center. Therefore, EF4 induces back-translocation by disengaging the tRNA’s CCA end (the head) from the peptidyl transferase center of the translating ribosome.
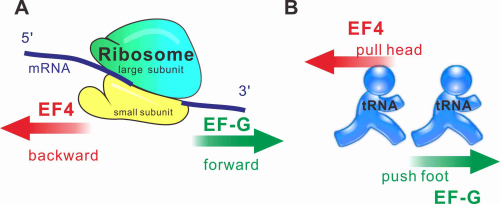
Mechanism of tRNA forward or backward movement catalyzed by EF-G and EF4, respectively. A, EF-G and EF4-catalyzed ribosomal movement along mRNA. B, EF-G catalyzes tRNA forward movement by pushing tRNA foot, EF4 catalyzes tRNA backward movement by pulling tRNA head (Image by QIN Yan)
Prof. QIN is supported by an IBP “One-Three-Five” Goal-oriented Project and the Key Laboratory of RNA Biology at IBP. This work was supported by grants from the Ministry of Science and Technology of China, the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences.
Contact:
QIN Yan
Institute of Biophysics, Chinese Academy of Sciences
http://www.ibp.cas.cn/ktzz/ktzz_QV/201308/t20130828_3920021.html
Email:qiny@ibp.ac.cn
GAO Ning

