Glutathionylation of the Bacterial Hsp70 Chaperone DnaK Provides a Link Between Oxidative Stress and the Heat Shock Response
Glutathionylation is a reversible modification of cysteine residues in proteins, which can protect proteins from irreversible oxidation, and can also play a role in signal transduction. Hsp70 is a highly conserved molecular chaperone essential for protein homeostasis and quality control. Cysteine modifications including glutathionylation have been reported in Hsp70 homologs from a wide range of organisms, suggesting a possible role in regulating the function of Hsp70.
In a collaboration between the groups of Prof. Sarah Perrett and Prof. CHEN Chang at Institute of Biophysics (IBP) of the Chinese Academy of Sciences, it was found that glutathionylation of the bacterial Hsp70 chaperone DnaK provides a link between oxidative stress and the heat shock response.
DnaK is the major bacterial Hsp70, participating in DNA replication, protein folding and the stress response. DnaK cooperates with the Hsp40 co-chaperone DnaJ and the nucleotide exchange factor GrpE. Under non-stress conditions, DnaK binds to the heat shock transcription factor Sigma32 and facilitates its degradation. Oxidative stress results in temporary inactivation of DnaK due to depletion of cellular ATP and thiol modifications such as glutathionylation, until normal cellular ATP levels and a reducing environment are restored. However, the biological significance of DnaK glutathionylation remains unknown, and the mechanisms by which glutathionylation may regulate the activity of DnaK are also unclear. We investigated the conditions under which Escherichia coli DnaK undergoes S-glutathionylation. They observed glutathionylation of DnaK in lysates of E. coli cells that had been subjected to oxidative stress. They also obtained homogeneously glutathionylated DnaK using purified DnaK in the apo state. They found that glutathionylation of DnaK reversibly changes the secondary structure and tertiary conformation, leading to reduced nucleotide and peptide binding ability. The chaperone activity of DnaK was reversibly down-regulated by glutathionylation, accompanying the structural changes. They found that interaction of DnaK with DnaJ, GrpE or Sigma32 becomes weaker when DnaK is glutathionylated and the interaction is restored upon deglutathionylation. This study confirms that glutathionylation down-regulates the functions of DnaK under oxidizing conditions, and this down-regulation may facilitate release of Sigma32 from its interaction with DnaK, thus triggering the heat shock response. Such a mechanism provides a link between oxidative stress and the heat shock response in bacteria.
This study entitled Glutathionylation of the Bacterial Hsp70 Chaperone DnaK Provides a Link Between Oxidative Stress and the Heat Shock Response was published in JBC on 25 March 2016.
The work was funded by the 973 Program of the Ministry of Science and Technology and the National Natural Science Foundation of China.
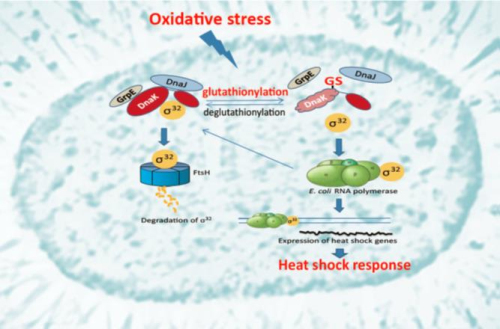
Legend: Glutathionylation of the bacterial Hsp70 chaperone DnaK provides a link between oxidative stress and the heat shock response (Image by IBP)
CONTACT:
Sarah Perrett or Chang Chen
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64889870 or 86-10-64888406
Email: sperrett@ibp.ac.cn or changchen@moon.ibp.ac.cn

