Study Unveils Novel Crosstalk Mechanism Between Mitochondrial Translation and Cytoplasmic Translation
Protein is the fundamental substance of life. The genetic code directing protein synthesis is stored in DNA. When a cell is instructed, the code information transfers from DNA to mRNA. Then, information on mRNA is further transferred to protein. There are two sets of protein translation system in mammalian cells, including the cytoplasmic translation system and the mitochondrial translation system, both of which are composed by ribosome, tRNAs and translation factors. The translation system translates mRNA into the biologically competent protein according to the information on mRNA. However, the coordination mechanism between the cytoplasmic translation system and the mitochondrial translation system has been a mystery to be solved.
A research article titled “Mammalian Elongation Factor 4 Regulates Mitochondrial Translation Essential for Spermatogenesis” was published online on the journal of Nature Structural & Molecular Biology on April 11, 2016, reporting the crosstalk mechanism between mitochondrial translation and cytoplasmic translation.
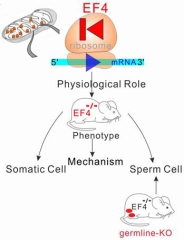
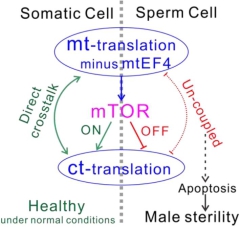
Mitochondrial translation elongation factor 4 (mtEF4) is a quality control factor of protein translation. Although the protein is highly conserved in evolution, previous mtEF4 gene knockout in some model organisms did not show significant phenotypic change. In this study, by using a systemic mtEF4 gene knockout mouse model, researchers found out that mtEF4 knockout damages the oxidative phosphorylation function in germ cells of the male mice, thus resulting in male sterility. Further study found that the rate of mitochondrial protein translation increased after mtEF4 was knocked out. However, the price is the lower protein "qualified rate" and the shorter protein half life. In order to keep step with the "quickened" mitochondrial translation, somatic cells activating the mTOR signaling pathway to accelerate cytoplasmic translation to balance the mitochondrial translation. In this way, somatic cells have successfully resolved the negative impact of the high-speed mitochondrial translation. In contrast, the mTOR signaling pathway could not be activated in germ line cells, cause the mitochondrial complex assembly of germ cells failed to assemble, and the sperm maturation process being stagnated at the round sperm stage, which ultimately resulted in the male sterility.
This study reveals a new information exchange way within the cell (see figure below): the mTOR signaling pathway balance the dynamic between mitochondrial translation and cytoplasmic translation. When the mitochondrial translation rate increases, the mTOR signaling pathway is activated, which caused the increase of cytoplasmic protein translation rate to counteract the pressure from the increased mitochondrial translation, which is a new evolutionary adaptation mechanism. Meanwhile, this study revealed a new way of male infertility, it is still of great value for the clinical treatment of male infertility.
The work is achieved by the cooperation of many institutions including: Institute of Biophysics (IBP), Institute of Zoology, Academy of Military Medical Sciences and Tianjin University of Science and Technology and other institutes. Institute of Biophysics and University of Chinese Academy of Sciences are the first and the second institutions, respectively. Prof. QIN Yan (IBP) is the corresponding author. GAO Yanyan and BAI Xiufeng are the co-first authors of this paper. This work is also supported by Chinese Ministry of Science and Technology, The National Natural Science Fund and Key projects of Chinese Academy of Sciences.
|
|
Schema: the crosstalk mechanism between mitochondrial translation and cytoplasmic translation (Image by QIN Yan's Group)
|
Contact:
QIN Yan
Institute of Biophysics, Chinese Academy of Sciences
http://www.ibp.cas.cn/ktzz/ktzz_QV/201308/t20130828_3920021.html
Email:qiny@ibp.ac.cn