Structural study of ISG54 reveals a novel RNA binding structure and potential functional mechanisms
Viral replication is the formation of biological viruses during the infection process in the target host cells. A virus can replicate only inside the living cells of an organism, i.e. the host cells. During replication, viruses generate abundant copies of its genome and package these copies into new viruses. Viruses depend on replication to survive and continue to infect new hosts. One important strategy of anti-viral drugs is to block viral replications. Study on the mechanisms how viral replications be blocked has become a hot spot in biomedical research.
Type I interferon (IFN) can block virus replication by stimulating the expression of various antiviral genes termed interferon-stimulated genes (ISG), including IFN-stimulated gene 56 family members (ISG56, also known as IFN-induced tetratricopeptide repeats, IFITs). The ISG56 family members play important roles in blocking viral replication and regulating cellular functions, however, their underlying molecular mechanisms are largely unclear.
Dr. LIU Yingfang at the Institute of Biophysics, Chinese Academy of Sciences has been working in the field of structural biology for more than 10 years in attempt to understand the mechanisms of cancer, viral replication and infection, and apoptosis by study the structure of related proteins.
In a recent study, Dr. LIU’s group reported in the Cell Research journal that ISG54, an ISG56 family protein with a novel RNA-binding structure. Structural study shows that ISG54 is a tetratricopeptide-repeat (TPR) containing protein. but unlike other known TPR proteins, it forms domain-swapped dimer. The C-terminal part of ISG54 folds into a super-helical structure by these TPR motifs and surprisingly has an extensively positively-charged nucleotide-binding channel on its inner surface, implying its possible nucleotide binding ability. EMSA results confirmed that ISG54 indeed binds specifically to some RNAs, such as adenylate uridylate (AU)-rich RNAs, with or without 5’triphosphorylation.
Mutagenesis and functional studies were then performed. These results show that the RNA binding ability is important to its antiviral activity. Disruption of RNA binding ability of this protein impairs its antiviral activity. In addition, they also identified that ISG54 can also bind some specific RNA sequence from cells such as ARE motif. Since proteins of ISG56 family have been suggested to be involved in some cellular functions, such as promoting apoptosis and inhibition of migration, their discoveries suggest a new mechanism not only for the antiviral activity of this interferon-inducible gene 56 family member but also for their potential cellular functions.
Supported by grants from the Ministry of Science and Technology and the National Science Foundation of China, this work may shed a new light for the functional mechanisms of this family proteins. A assay published in Cell Research recently highlighted their results as a breakthrough in the study of these family proteins.
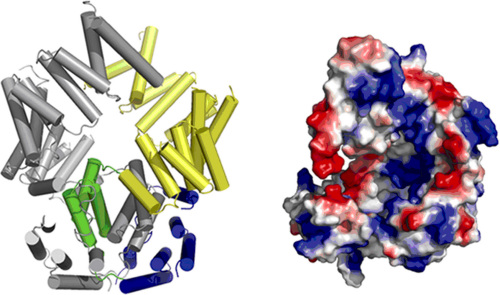
Figure 1. Overall structure of ISG54. (A) Cartoon diagram of the ISG54 dimer. The N-terminal region (blue), domain-swapped region (green) and C-terminal region (yellow) are shown in monomer A and monomer B is in gray. (B) The ISG54 structure shows a nucleotide-binding region. The electrostatic potential surface of monomer A is shown in the right panel (red, negative charge; blue, positive charge; white, neutral). From LIU Yingfang et.al.

