Breakthrough in the kinase activity regulation mechanism of PI4KIIα
Kinase is a type of enzyme that catalyzes the transfer of phosphate groups from a high-energy phosphate-containing molecule (as ATP) to a substrate. Kinases are critical in metabolism, cell signalling, protein regulation, cellular transport, secretory processes, and countless other cellular pathways.
Phosphatidylinositol (PI) 4-kinase IIα (PI4KIIα), the most abundant PI4K in mammalian cells, is best known for its essential role in providing the substrate for phosphatidylinositol 3-kinase (PI3K) and the production of second messengers (DAG and InsP3). PI4KIIα also plays important roles in its own right in membrane trafficking, receptor signaling transduction, phagocytosis and the exo-endocytic cycle of synaptic vesicles. Its dysfunction contributes to tumor growth, spastic paraplegia and Gaucher’s disease. Therefore, PI4KIIα is a potential important drug target.
Professor CHEN Chang at the Institute of Biophysics, Chinese Academy of Sciences studies the function of PI4KIIα in different diseases especially in cancer. She firstly demonstrated that suppression ofPI4KIIαcan inhibit tumor growth. Recently she collaborated with another IBP professor, SUN Fei, and Professor Klaus Schulten at University of Illinois at Urbana-Champaign, USA. They resolved the crystal structure of human PI4KIIα in ADP-bound form, which is the first crystal structure in the PI4Ks family.
In this study, three novel insertions that are not present in other PIKs were found in PI4KIIα, namely I1 (palmitoylation insertion) and I2(RK-rich insertion) in the N-lobe and I3 in the C-lobe. The palmitoylation insertion and RK-rich insertion are critical for the regulation of PI4KIIα’s membrane binding and kinase activity. More excitingly, based on the structure, they found a distinct nucleotide-binding pocket of PI4KIIα that differs notably from that of PI3Ks, which well explained the insensitivity of PI4KIIα to PI3Ks’ inhibitors.
Although many PIKs crystal structures had been resolved, the exact substrate binding pockets was still not clear. By combining molecular dynamics (MD) simulations method, Professor CHEN and her colleagues identified putative PI-binding pocket formed both by activation loop and palmitoylation insertion.MD stimulation, biochemical and mutagenesis studies reveal that any perturbation of the interaction between PI4KIIα and membrane will affect either the nucleotide binding or PI binding and then modulate the kinase activity of PI4KIIα. In particular, change of the membrane environment via altering lipid composition or through adding specific chemical compounds should regulate the kinase activity of PI4KIIα.
Determination of the crystal structure of human PI4KIIα catalytic domainhas provided molecular insight into how the kinase activity of PI4KIIαcan be regulated via changing membrane organization, such as cholesterol. This achievement lays the foundation for designing specific PI4KIIα’s inhibitors and activators for future application in related diseases. Moreover, the explored kinase activity regulation mechanism offers a new concept for drug design viadeveloping compounds that modulate the membrane environment to regulate the activity of the target. This breakthrough is not only of scientific importance to biologists and medical researchers; it also attracted great interests from pharmacological industry to develop novel drugs.
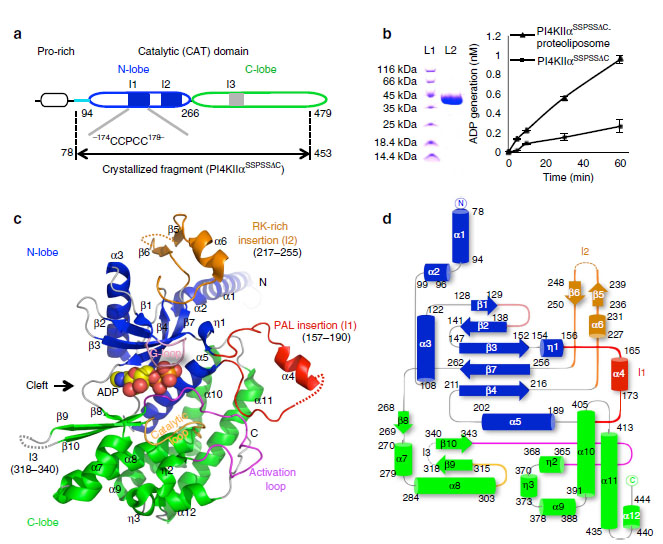
Figure 1.Overall structure of the human PI4KIIa catalytic domain. (from NATURE COMMUNICATIONS | 5:3552 | DOI: 10.1038/ncomms4552)
The researchers published an article entitled “Molecular insights into the membrane-associatedphosphatidylinositol 4-kinase IIa” in the latest issue of the Nature Communications journal introducing this work.
This work was supported by grants from the Ministry of Science and Technology of China, the ‘863’ National High-Technology Development Program, and the National
Natural Science Foundation of China.

