A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71
Enterovirus 71 (EV71) is the main causative virus of the widespread Hand-foot-and-mouth Disease (HFMD). In China, more than 4 million cases and over 2 thousands deaths were reported in recent four years, but no vaccine or antiviral therapy is available at present. Structural analysis of the deadly virus may help us better understand its pathological mechanism and reveal new opportunities for therapeutic intervention.
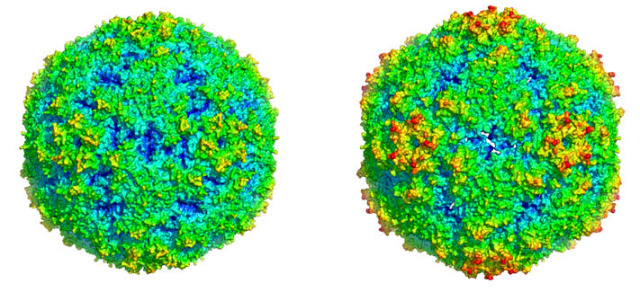
Professor RAO Zihe and his research group at the Institute of Biophysics, Chinese Academy of Sciences recently reported their structural analysis of EV71 virus capsid in the journal of Nature Structural and Molecular Biology. In collaboration with Professor Wang Junzhi at the National Institutes for Food and Drug Control and Professor David Stuart at Oxford University, they resolved the crystal structure with high resolution of mature full particle of EV71, as well as that of one kind of its pre-mature empty particle. Compared with the mature full particle, the empty particle has notable openings on the two-fold and typical canyon of its capsid, with the diameter of the particle expand about 4%. Hydrophobic pockets in the EV71 capsid are collapsed in this expanded particle, providing a detailed explanation of the mechanism for receptor-binding triggered virus uncoating. The new structural knowledge provided by this study might facilitate the development of generic therapies against enteroviruses, some of which, for instance EV71, represent serious threats to human and/or animal health.

