Structural study of TTR-52 reveals the mechanism by which a bridging molecule mediates apoptotic cell engulfment
Phagocytosis and the removal of apoptotic cells are important events in tissue remodeling, suppression of inflammation, and regulation of immune responses. Defects in these processes cause autoimmune diseases and developmental retardation.
During apoptosis, apoptotic cells are marked by “eat me” signals such as phosphatidylserine (PtdSer), which are exposed on the surface of apoptotic cells. The apoptotic cells are removed by professional phagocytes or neighboring engulfing cells either directly through phagocytic receptors or indirectly through bridging molecules that cross-link dying cells to phagocytes. However, how bridging molecules recognize ‘‘eat me’’ signals and phagocytic receptors to mediate engulfment remains unclear.
In a recent report [1], professor LIU Yingfang’s group at the Institute of Biophysics, Chinese Academy of Sciences and Professor WANG Xiaochen’s group at the National Institute of Biological Sciences, Beijing, reported the structural and functional studies of Caenorhabditis elegans TTR-52, a recently identified bridging molecule that cross-links surface-exposed phosphatidylserine (PtdSer) on apoptotic cells to the CED-1 receptor on phagocytes.
Crystal structure studies show that TTR-52 has an open β-barrel-like structure with some similarities to the PKCa-C2 domain. TTR-52 is proposed to bind PtdSer via an ‘‘ion-mediating’’ PtdSer-binding mode. Intensive functional studies show that CED-1 binds TTR-52 through its N-terminal EMI domain and that the hydrophobic region of the TTR-52 C terminus is involved in this interaction. In addition, unlike other PtdSer-binding domains, TTR-52 forms dimers, and its dimerization is important for its function in vivo.
These results revealed the first full-length structure of a bridging molecule and the mechanism underlying bridging molecule-mediated apoptotic cell recognition. This work was supported by grants from the Ministry of Science and Technology and the National Science Foundation of China.
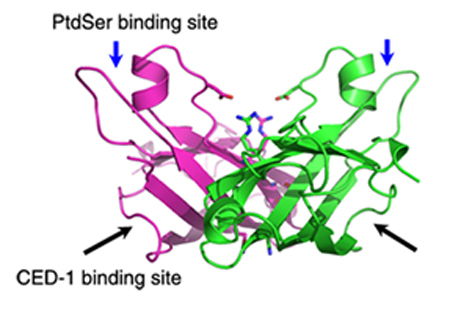
Fig. The interface of the TTR-52 (M5) dimer shown as a cartoon. The proposed PtdSer- and CED-1-binding sites are indicated as blue and black arrows, respectively. (from LIU Yingfang et.al)
[1] Yanyong Kang, Dongfeng Zhao, Huanhuan Liang, Bin Liu, Yan Zhang, Qinwen Liu, Xiaochen Wang, and Yingfang Liu. Structural study of TTR-52 reveals the mechanism by which a bridging molecule mediates apoptotic cell engulfment. (2012) GENES & DEVELOPMENT 26:1339–1350

