A Drosophila model for TDP-43 proteinopathy
TDP-43 is a protein linked to a range of neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS), frontotemporal lobar dementia (FTLD), Alzheimer’s disease (AD), and other types of dementia. In a paper published recently in the Proceedings of the National Academy of Sciences1 Professors Yan Li, Jane Wu and their colleagues constructed a Drosophila model expressing human TDP-43 in order to investigate its role in pathogenesis.
Simply overexpressing hTDP-43 in different types of neurons was sufficient to cause protein aggregation and neuronal loss in an age-dependent manner. Expression in fly eyes of full-length hTDP-43, but not a mutant lacking its amino-terminal domain, led to progressive loss of ommatidia with marked signs of neurodegeneration, suggesting that the RNA recognition motif is important in pathogenesis. Expressing hTDP-43 in mushroom bodies resulted in dramatic axon losses and neuronal death. When hTDP-43 was expressed in motor neurons, it led to axon swelling, reduction in axon branches and bouton numbers, and ultimately to motor neuron loss and motility defects. TDP-43 is normally localized in the nucleus, however, Professor Li and colleagues found that when TDP-43 was located in the cytoplasm, cells displayed swelling, nuclear fragmentation, and cell death, thus linking aberrant cellular accumulation with pathogenesis.
Results from parallel studies of flies lacking Drosophila TDP demonstrated that an insufficient level of TDP also induces axon loss, neuronal death, and locomotion impairment, but in a different manner to that in TDP overexpressing flies. These results indicated that an appropriate level of TDP is critical for normal neural development and function, and that bidirectional changes cause different subtypes of TDP proteinopathy. If these findings also apply to human diseases, mutations of TDP-43 in different patients may lead to increases or decreases in the protein level, thus resulting in different phenotypes. Examining and adjusting the level of TDP-43 may be a useful therapeutic strategy to alleviate symptoms.
This new Drosophila model exhibits many of the important pathological and clinical features of ALS and will thus be a powerful tool for investigating TDP-43-related diseases. It can be used both to identify genes that interact with TDP-43 and to test drugs that might reduce protein toxicity.
1 Li et al. (2010) A Drosophila model for TDP-43 proteinopathy. PNAS 107(7): 3169-3174
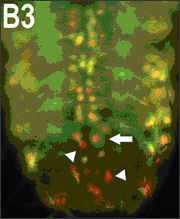 |
Fig1. Expression of human TDP-43 in transgenic Drosophila causes morphological and functional defects of motor neurons (MNs).MNs expressing hTDP-43 showed cell death and morphological defects. The arrow marks a swollen neuron and the arrowheads mark MNs with fragmented or condensed nuclei. |
|
|
|
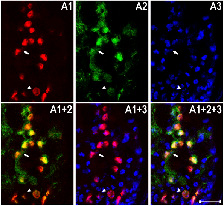
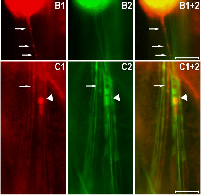
Fig2. Overexpression of human TDP-43 in motor neurons leads to protein aggregation in cytoplasm and axons, together with axonal swelling. (A and B) Overexpressing hTDP-43 using the motor neuron-specific driver (OK371-Gal4) resulted in protein aggregates detectable in the cytoplasm (A) and axons (B). Arrowheads in A1–A3, A1+2, A1+3, and A1+2+3 mark cells which contain a condensed and fragmented nucleus. Arrows in B1 and B1+2 mark hTDP-43-containing abnormal axonal varicosities. (C) All hTDP-43-overexpressing flies exhibited axon swelling with TDP-43-positive inclusions, which was absent in control flies. Some regions with TDP-43 protein aggregates show reduced mGFP signals (marked by arrows), and other regions exhibit significant axonal swelling as marked by arrowheads. In all images: red, hTDP-43-RFP; green, mGFP; blue, Hoechst staining. (Scale bars: 50 μm.)