MTMR4 Dephosphorylates R-Smads in Early Endosomes
The ancient Chinese theory of “Yin-Yang” holds that all things are in balance, and biological signaling is no exception. The TGFβ signaling pathway plays a fundamental role in the development and physiology of a diverse range of animal species. Phosphorylation and dephosphorylation of R-Smads is known to be a crucial regulatory mechanism for maintaining the homeostasis of TGFβ signaling. Although phosphatases which dephosphorylate R-Smads in the nucleus have been well studied, it has been unclear whether there are any cytoplasmic phosphatases that can attenuate R-Smads phosphorylation and nuclear translocation. In a recent paper published in the Journal of Biological Chemistry1, Professor Hong Tang’s lab reports for the first time that MTMR4, a cytoplasmic phosphatase, plays a significant role in TGFβ signaling by specifically reducing the phosphorylation level of R-Smads in early endosomes.
Results from co-immunoprecipitation experiments showed that endogenous MTMR4 interacts with phosphorylated R-Smads, and that this interaction is correlated with the dephosphorylation of R-Smads.Further analysis showed that overexpression of MTMR4 resulted in the sequestration of activated Smad3 in early endosomes, thus reducing its nuclear translocation. Both point mutations at the conserved catalytic site of this phosphatase, and siRNA knockdown of endogenous Mtmr4 expression led to sustained Smad3 activation. Professor Tang’s group concluded that MTMR4 plays an important role in preventing the overactivation of TGFβ signaling by dephosphorylating activated R-Smads that have been trafficked to early endosomes.
1 Junjing Yu, Lei Pan, Xincheng Qin, Hua Chen, Youli Xu, Yeguang Chen, and Hong Tang (2010): MTMR4 Attenuates Transforming Growth Factor β (TGFβ) Signaling by Dephosphorylating R-Smads in Endosomes
The Journal of Biological Chemistry 285 (11): 8454–8462.
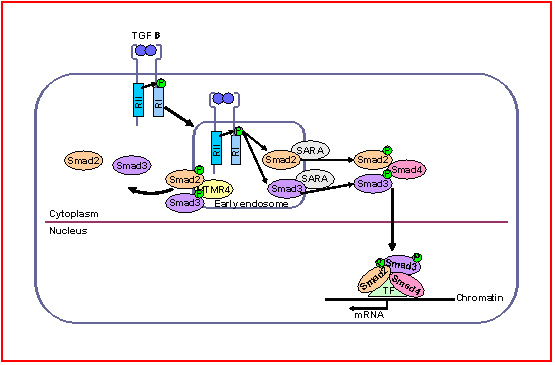
MTMR4 serves as a phosphotase in the early endosome to dephosphorylate activated R-Smads.

