Researchers discovered a Golgi-derived vesicle regulating endosome fission
The endosome has highly dynamic motility and frequently undergoes fission and fusion events to coordinate endocytic trafficking. Endosome fission has been reported to be associated with cargo sorting. After internalization, cargoes can be segregated into distinct domains allocated for their destination, proceeding to either lysosomes for degradation, or the plasma membrane or trans-Golgi network (TGN) for recycling. Until now, the endoplasmic reticulum (ER) network has been recognized as the central regulator that defines where and when endosomes undergo fission. However, whether and how other organelles participate in endosome fission is unknown.
On 28th June of 2021, Associate investigator JIA Shunji (Professor MENG Anming's group in School of Life Sciences, Tsinghua University), and Professor LI Dong's group (National Laboratory of Biomarcomolecules, Institute of Biophysics) published a research article on Nature Cell Biology, entitled "A Golgi-derived vesicle potentiates PtdIns4P to PtdIns3P conversion for endosome fission". This article reported a novel function of the Golgi apparatus derived SEC14L2 compartment in regulating the ER-associated endosome fission. This paper demonstrates the coordinated interactions among distinct intracellular membrane systems are crucial for precisely executing the biological function.
In this study, researchers characterized a Golgi-derived vesicle, named as the SEC14L2 compartment, through the combination of super-resolution live-cell imaging system with the EM technology. When the inhibitor BFA was applied to block the formation of this vesicle, the ER-associated endosome fission is significantly delayed, causing endosomes with enlarged and tubulated morphology. This phenomenon indicates a potential role of the Golgi apparatus involved in the ER-assisted endosome fission. To observe the dynamics of SEC14L2 compartment, the ER and endosomes, researchers employed the grazing incidence structured illumination microscopy (GI-SIM), and found that the SEC14L2 compartment frequently contacted with the ER and endosomes. More interestingly, during endosome fission, the SEC14L2 compartment was recruited and stabilized onto the dividing site where the ER tubule across. Meanwhile, the SEC14L2 compartment facilitated the PtdIns3P accumulation and PtdIns4P removal on endosome membrane. Only after endosomal PtdIns4P to PtdIns3P conversion was done, an endosome proceeded its division.
Mechanically, SEC14L2 protein enriched in the Golgi-derived vesicle, plays a cardinal role in regulation endosome fission. On one hand, SEC14L2 directly binds PtdIns3P and PtdIns4P that directs their transfer among liposomes in vitro. On the other hand, SEC14L2 interacts with the PI3K and activates it to promote PtdIns3P generation. When knocking out SEC14L2 protein in the COS-7 cells, it shows a significant reduction of endosomal PtdIns3P level and abnormal accumulation of endosome PtdIns4P, which results in delayed endosome fission and enlarged endosome, consistent with observations upon depletion of the SEC14L2 compartment. To validate involvements of SEC14L2's lipid binding activity in endosome fission, researchers performed the rescue experiments and found that the lipid-binding deficient form, SEC14L2-M5, failed to rescue endosome fission defects in the SEC14L2 KO COS-7 cells, in contrast wild-type SEC14L2 form successfully rescue this phenotype. Taken together, it was concluded that the lipid-binding activity is essential for SEC14L2-mediated endosome fission. It's worth noting that in the process of submitting this work, a study published on Science, also reports the function of the Golgi-derived vesicle in regulating the ER-associated mitochondrial fission. Both studies highlight an unanticipated role of the Golgi network in intracellular membrane homeostasis, although it requires further work to demonstrate the identity of these Golgi-derived vesicles.
Associated investigator JIA Shunji from School of Life Sciences of Tsinghua University and Professor LI Dong from Institute of Biophysics, Chinese Academy of Sciences are the correspondence authors of the article. Post-doc associate GONG Bo from Tsinghua University and GUO Yuting from Institute of Biophysics, Chinese Academy of Sciences are the co-first authors of the article. Other members contributed to the article are listed below: Ph.D. student DING Shihui from School of Life Science of Tsinghua University, Associated investigator LIU Xiaohui from School of Life Sciences, National Protein Science Facility, Tsinghua University and Professor MENG Anming from State Key Laboratory of Membrane Biology, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University.
This work was supported by the grants from the National Natural Science Foundation of China, Ministry of Science and Technology of China, National Key Research and Development Program of China, and China Postdoctoral Science Foundation (#91754112, #91754202, #2019YFA0801403, #31801130, #32000480, and BX20190355).
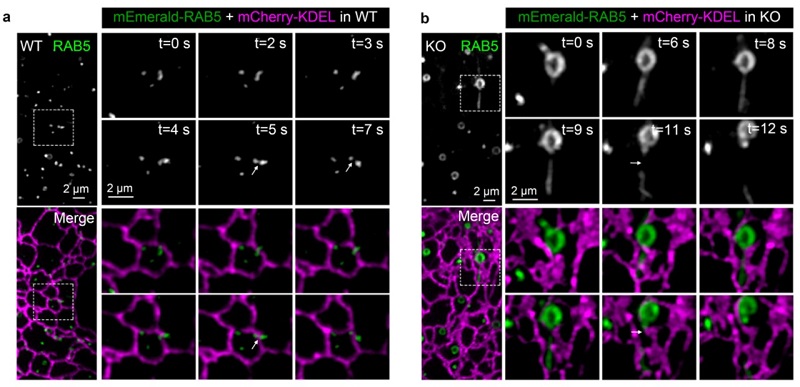
Fig 1. Compared to WT COS-7 cells (a), the ER-associated endosome fission in SEC14L2 KO COS-7 cells (b) is significantly delayed, causing endosomes with enlarged size.
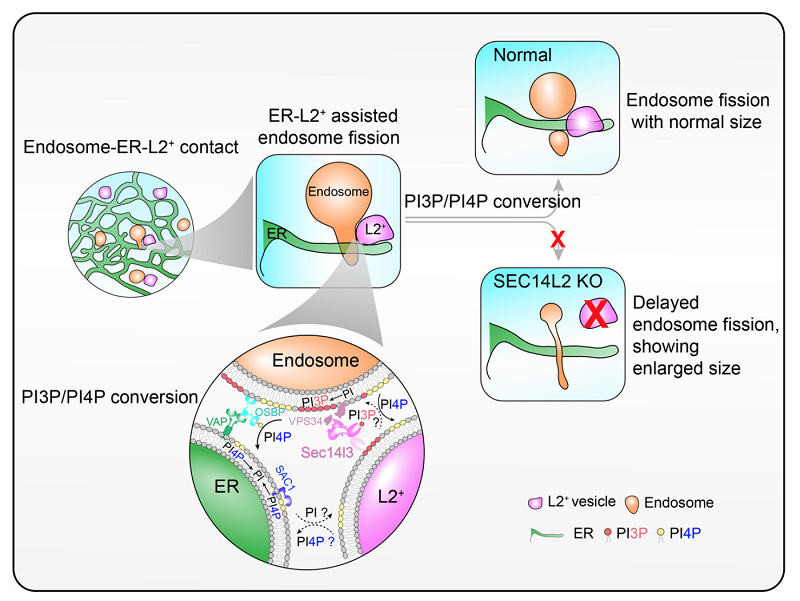
Fig 2. Working model of the SEC14L2 compartment in the ER-associated endosome fission. During endosome fission, the SEC14L2 compartment is recruited to the fission site marked by the ER tubules to promote PI4P to PI3P conversion on endosomes.
Paper link:https://www.nature.com/articles/s41556-021-00704-y
Contact: LI Dong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: lidong@ibp.ac.cn
(Reported by Dr. LI Dong's group)

