Supramolecular structural basis for state transitions of photosynthesis in green algae
Green algae are photosynthetic organisms commonly found in aquatic and soil environments, fulfilling important roles as primary producers in the ecosystem. During photosynthesis, they can absorb light energy through the light-harvesting complexes I and II (LHCI and LHCII) to drive the subsequent charge separation and energy conversion processes occurring in photosystems I and II (PSI and PSII, similar to those found in plants). As PSI and PSII exhibit different absorption characteristics, imbalance of energy distribution between the two photosystems tends to occur in natural environments, where light quality and quantity fluctuate constantly.
State transitions are a short-term light acclimation mechanism serving to balance energy distribution between two photosystems. When PSII is over-excited, its peripheral antenna, light-harvesting complex LHCII, is phosphorylated, and a portion of the phosphorylated LHCIIs migrates from PSII to PSI, forming the PSI-LHCI-LHCII supercomplex. Thereby, the photon energy collected by LHCII can be transferred to the PSI core to increase its excitation level.
In green algae, state transitions show stronger amplitude and play a wider photoprotective role than the process occurring in plants. In the model green alga Chlamydomonas reinhardtii, the PSI-LHCI-LHCII (CrPSI-LHCI-LHCII) supercomplex is larger and more intricate when compared to the one from plants, with at least two LHCII trimers associated with the PSI core. Moreover, the composition of CrLHCII trimers is also complicated, as there are 9 different LhcbM proteins (LhcbM1-9), which are classified into four types (types I-IV), participating in the formation of LHCII trimers in C. reinhardtii.
The detailed structure of CrPSI-LHCI-LHCII supercomplex and the subunit composition of LHCII trimers associated with PSI are still unclear. Fundamental questions, as to which LhcbM isoforms bind to green algal PSI and how two LHCII trimers establish specific interactions with PSI, remain unresolved. The potential excitation energy transfer (EET) pathways between LHCII and PSI-LHCI are to be unraveled at high resolution.
Recently, LI Mei's group and LIU Zhenfeng's group from the Institute of Biophysics (IBP) at CAS, in collaboration with Jun Minagawa's group from National Institutes for Basic Biology, Japan, have solved the high-resolution cryo-electron microscopy (cryo-EM) structures of CrPSI-LHCI-LHCII supercomplex. The team discovered that the supercomplex is composed of a PSI core, 8 LHCI proteins bound to PSI on one side, 2 LHCI monomers and 2 LHCII trimers associated with PSI on the other side. Collectively, they form a membrane protein-pigment supramolecular complex with an overall molecular weight of about 1,100 kDa (Fig. a).
Based on the high-quality electron microscopy density, the LhcbM proteins involved in the formation of the CrPSI-LHCI-LHCII supercomplex have been identified, and it was found that the supercomplex contains all four types of LhcbM proteins. Two specific LhcbM isoforms, namely LhcbM1 and LhcbM5, directly interact with the PSI core through their phosphorylated amino-terminal regions (Fig. b-c). In addition, LhcbM5 further serve as a connector to stabilize the assembly between LhcbM1 and the PSI core. Furthermore, biochemical and functional studies on mutant strains lacking either LhcbM1 (ΔLhcbM1) or LhcbM5 (ΔLhcbM5) indicate that only LhcbM5 is indispensable for the formation of PSI-LHCI-LHCII supercomplex, and the state transitions in ΔLhcbM5 mutant are affected by the lack of LhcbM5 (Fig. d-e). In the ΔLhcbM1 mutant, LhcbM3 protein replaces LhcbM1 and binds to PSI, thus the supercomplex is still present in this mutant strain. The specific interactions and potential excitation energy transfer routes between green algal PSI and two phosphorylated LHCIIs have been unraveled in great details, and provide an important foundation for understanding the molecular mechanism and evolution of photosynthetic state transitions.
The research work, entitled "Structural basis of LhcbM5-mediated state transitions in green algae", was published in Nature Plants on July. 8, 2021. The project was supported by grants from the Ministry of Science and Technology of China, National Natural Science Foundation of China and Chinese Academy of Sciences.
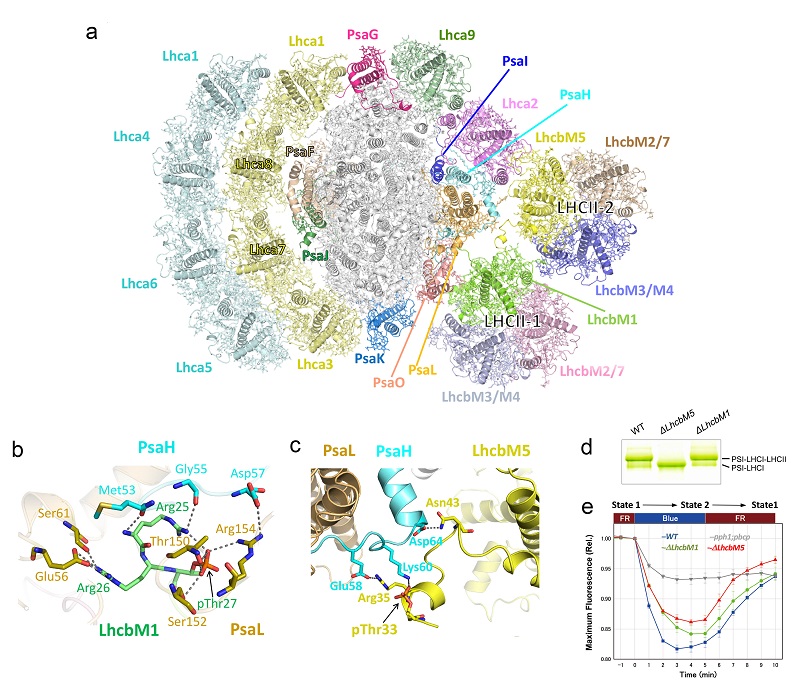
Figure: The PSI-LHCI-LHCII supercomplex from Chlamydomonas reinhardtii
Paper link:https://www.nature.com/articles/s41477-021-00960-8
Contact: LI Mei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: meili@ibp.ac.cn
(Reported by Dr. LI Mei's group)

