Dr. ZHU Bing's and Dr. XU Rui-Ming's groups collaboratively uncovered the function of BEND3, a CpG island (CGI) binding protein, in transcriptional regulation of bivalent genes during differentiation
On February 10, 2022, a cooperative study between the groups of Drs. ZHU Bing and XU Rui-Ming from the Institute of Biophysics, Chinese Academy of Sciences was published in Science online, entitled "Highly enriched BEND3 prevents the premature activation of bivalent genes during differentiation". In this study, the researchers report a reining function of BEND3 at its highly occupied bivalent genes in ES cells to prevent them from premature activation during differentiation.
In vertebrate genome, approximately 70% of gene promoters are associated with DNA elements called CpG islands (CGIs), which are characterized by high density of CpG dinucleotides and lack of DNA methylation. CGIs are not only associated with housekeeping genes, but also with bivalent genes. A recent study by Dr. Dirk Schübeler's group reported that BANP, a DNA methylation-sensitive CGI binding protein, binds CGCG element and activates CpG-island-regulated essential metabolic genes. It is intriguing to ask whether and how CGI-associated bivalent genes are regulated during development by sequence-specific transcription factors targeting CGIs. In a previous study, Dr. ZHU Bing's group identified BEND3 as a protein preferentially associating with unmethylated DNA in vitro, which suggesting BEND3 may recognize non-methylated CGIs in vivo. However, little was known about the genomic distribution and physiological function of BEND3.
In this study, the researchers performed ChIP-seq experiments and found that BEND3 is enriched at regulatory elements associated with CGIs, including promoters and enhancers. They determined the binding motif of BEND3 and solved the cocrystal structure of the critical fourth BEN domain in complex with its target DNA.
To investigate the physiological roles of BEND3, they performed mouse experiments and found mouse embryos depleted of Bend3 died around peri-gastrulation stage. Consistently, Bend3 KO ES cells exhibited severe defects in differentiation, both in teratoma and embryoid body (EB) formation assays. To clarify the molecular mechanism underlying the developmental defect, the researchers performed RNA-seq experiments of EB differentiation process. Gene expression profiling revealed that hundreds of CGI-containing bivalent genes were activated prematurely in Bend3 null mutants. Furthermore, bivalent genes with high level of BEND3 binding and more BEND3 binding motifs showed the most significant transcriptional changes.
It was reported that BEND3 participates in the recruitment of PRC2 to major satellites in cells lacking DNA methylation. Therefore, the researchers performed ChIP-seq experiments for SUZ12 (a subunit of PRC2) and H3K27me3, and demonstrated that BEND3 is required for stable association of PRC2 complex and maintaining high level of H3K27me3 at some bivalent genes highly enriched with BEND3.
Taken together, this study identified a novel CGI binding protein, BEND3, which is required for the optimal association of PRC2 at bivalent genes to prevent these genes from premature activation during differentiation. CGIs have long been considered as regulatory elements of transcription. This work offers highly interesting new insights into the critical roles of CGIs and CGI-binding proteins.
Gene bivalency is widely expected in priming for faster induction, largely due to the enrichment of H3K4me3. However, H3K4me3 removal by MLL2 deletion at bivalent genes didn't affect their induction kinetics. Bend3 deletion leads to reduced PRC2 and H3K27me3 levels at its highly enriched bivalent genes. This does not immediately activate the majority of these genes, but affects the kinetics of these bivalent gene induction. This study proposes that gene bivalency can prevent premature gene activation during differentiation, which is an opposite effect of priming, and the authors term it as reining.
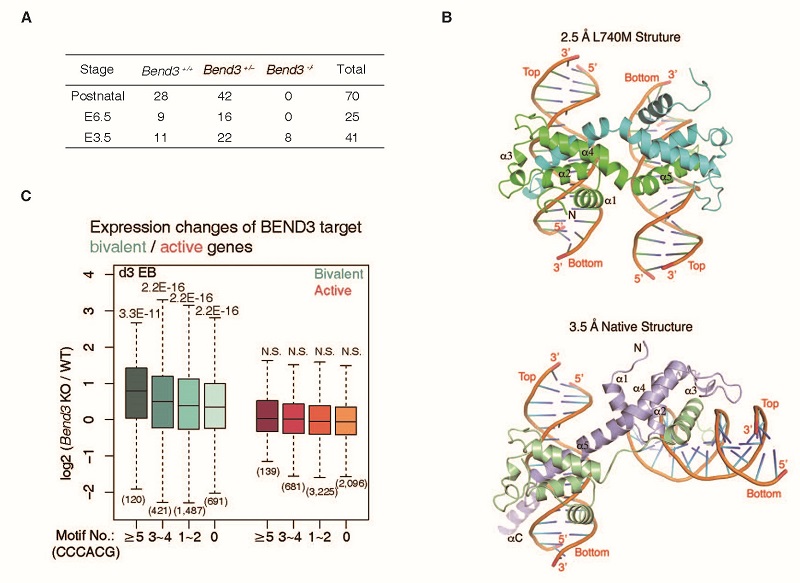
Figure:BEND3 is essential for development and differentiation.
(Image provided by Dr. ZHU Bing's group)
Drs. ZHU Bing and XU Rui-Ming are corresponding authors. Drs. ZHANG Jing, ZHANG Yan and YOU Qinglong are equal contributing first authors. This study was supported by funds from the Ministry of Science and Technology of China, National Natural Science Foundation of China and the Chinese Academy of Sciences.
The web link of this paper: https://www.science.org/doi/10.1126/science.abm0730
Contact: ZHU Bing
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64888832
Email: zhubing@ibp.ac.cn
(Reported by Dr. ZHU Bing's group)

