Structural basis for the photosynthetic cyclic electron transport in plants
Photosynthetic electron transport is driven by light energy and can be classified into two types, the linear electron transport/flow (LET/LEF) and the cyclic electron transport/flow (CET/CEF). The LEF pathway involves both photosystems I and II (PSI and PSII) as well as cytochrome b6f (Cytb6f), and produces ATP and NADPH, which are utilized in dark metabolic reactions. As for the CEF pathway, electrons are recycled between Cytb6f and PSI without producing NADPH, while the proton gradient across the thylakoid membrane is generated and drives ATP synthesis. CEF is essential for optimizing the growth and fitness of phototrophs.
In plants and cyanobacteria, type-I NADH dehydrogenase-like (NDH) complex is crucial for CEF pathway, mediating the electron transport between PSI and Cytb6f. NDH is a multi-subunit membrane protein complex. Cyanobacterial NDH-1L contains 19 protein subunits, while plant NDH consists of at least 29 subunits. In addition, photosynthetic NDH is able to associate with PSI to form NDH-PSI supercomplex, which increases the stability of NDH complex and facilitates the CEF process.
To investigate the assembly details and working mechanism of plant NDH-PSI supercomplex, the research team in Institute of Biophysics, Chinese Academy of Sciences solved the 3.9 Å resolution structure of the NDH-PSI supercomplex from Arabidopsis thaliana. The work entitled with "Supramolecular Assembly of Chloroplast NADH dehydrogenase-like complex with photosystem I from Arabidopsis thaliana" is published in《Molecular Plant》on March 7th, 2022.
In the supercomplex, one chloroplast NDH is sandwiched by two PSIs, a total of 58 protein subunits and 415 cofactors (Figure A) were modeled in the structure. Plant NDH contains 29 subunits belonging to five subcomplexes. Except three subunits of SubE, 26 NDH subunits were modeled in the structure (Figure B). The two PSI complexes adopt overall architectures highly similar to the free PSI reported previously, with substitution of one peripheral light-harvesting complex I (LHCI) in each PSI, namely, Lhca2 is replaced by Lhca6 in one PSI, and Lhca4 is replaced by Lhca5 in the other PSI. The structure revealed the crucial roles of Lhca6 and Lhca5 in mediating the interaction between PSI and NDH, and showed that Lhca6 and Lhca5 bind fewer pigments than Lhca2 and Lhca4. The result suggested that during evolution, plants sacrifice their light-harvesting capability to form NDH-PSI supercomplex for better CEF performance. Based on the structural information, a working model of NDH dependent CEF (Figure C) is proposed.
Professors LI Mei, ZHANG Xinzheng from Institute of Biophysics, CAS, and PAN Xiaowei from Capital Normal University are corresponding authors. Associate professors SU Xiaodong and CAO Duanfang are co-first authors. The project was funded by Strategic Priority Research Program of the CAS, the National Natural Science Foundation of China, the CAS Project for Young Scientists in Basic Research, the National Key R&D Program of China, and the Youth Innovation Promotion Association at the CAS. Data collection and sample analysis are supported and assisted by the "Center for Biological Imaging" and Protein Science Research platform of the Institute of Biophysics, CAS.
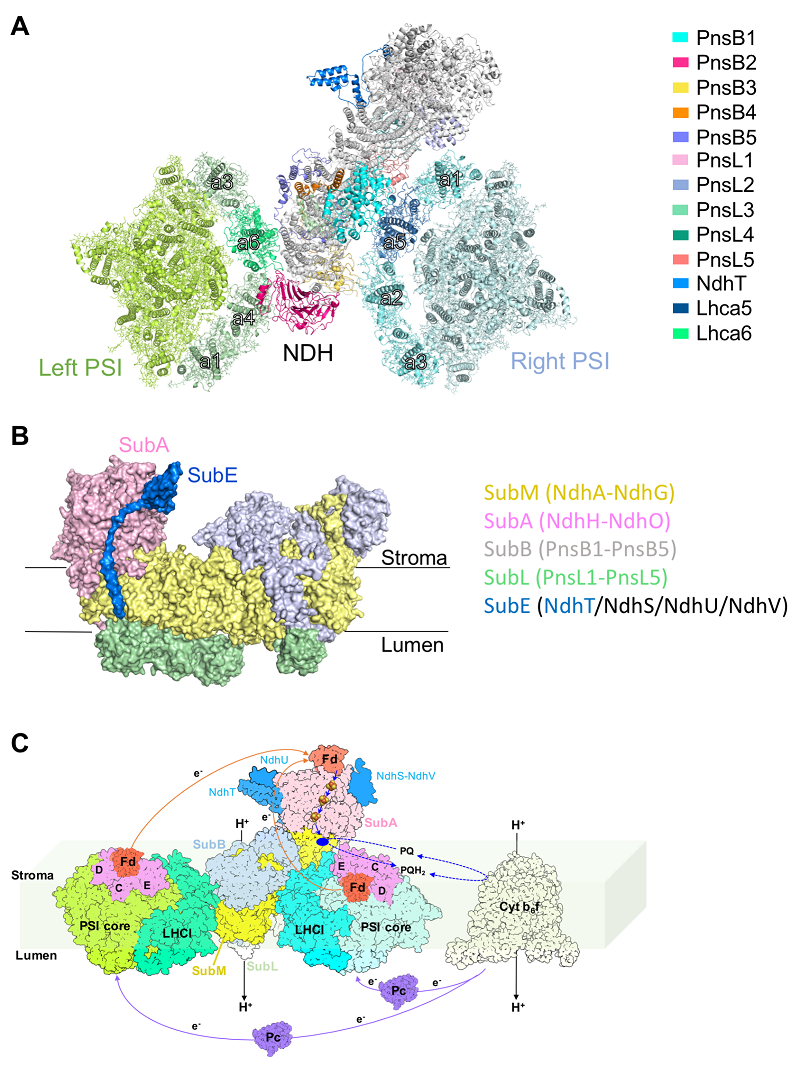
Figure. Structure and schematic model of NDH-PSI supercomplex.
A. Overall structure of NDH-PSI; B. Structure of NDH; C. A schematic model of cyclic electron transport in plants.
The web link of this paper: https://www.sciencedirect.com/science/article/pii/S1674205222000478
Contact: LI Mei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: meili@ibp.ac.cn
(Reported by Dr. LI Mei's group)

