Research reveals mechanism of polarized cortex assembly in migrating cells
The cell cortex is defined as a thin layer of filamentous actin, myosin motors, and regulatory proteins beneath the plasma membrane. Assembly and contraction of this actin meshwork generates cortical tension, which enables cells to resist external mechanical stresses, change shape, and exert forces. Consequently, the cortex plays a critical role in a variety of cellular processes, including division, migration, and morphogenesis.
The mechanical properties of the cortex are key to its physiological function. Changes in cortical mechanics can originate from changes in the architecture of the actin network. However, the complete inventory of assembly factors driving formation of the actin cortex and how their activities are spatiotemporally controlled are not well understood.
In a recent study published in Journal of Cell Biology, Professor CAI Huaqing's group at the Institute of Biophysics Chinese Academy of Sciences uncovered a signaling cascade responsible for building the rear actin cortical subcompartment in rapidly migrating cells.
Using Dictyostelium as a model for polarized and rapidly migrating cells, they found that a RhoGEF domain-containing protein named GxcM localizes specifically in the rear of directionally migrating cells. Overexpression of GxcM induces excessive actin polymerization in the rear cortex, and these structures can be marked by the Arp2/3 complex, suggesting that GxcM possesses the ability to promote branched actin assembly. By generating a series of truncations and mutations, they demonstrated that the function of GxcM relies on its C-terminal proline-rich motifs and GEF activity.
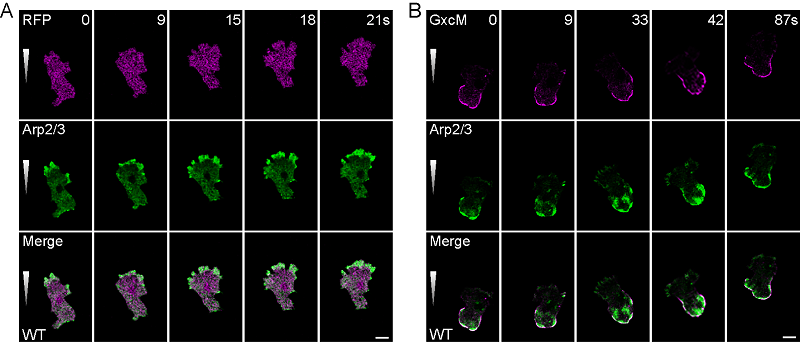
Fig 1. GxcM localizes specifically in the rear of migrating cells, promoting Arp2/3 complex-based actin assembly.
On the one hand, co-immunoprecipitation, mass spectrometry, and microscopic imaging experiments showed that GxcM binds to and recruits F-BAR protein, Fbp17, through its C-terminus. In fbp17 knockout cells, GxcM still localizes in the posterior of the cell, but loses the ability to stimulate actin polymerization, resulting in a decrease in cortical actin content, a significant reduction in the cell's ability to resist external mechanical forces, and defects in cell division and migration. Biochemical and in vitro actin polymerization experiments showed that Fbp17 is capable of binding and activating the actin nucleation promoting factor WASP through its SH3 domain, which in turn activates Arp2/3 and mediates branched actin polymerization.
On the other hand, they found that GxcM regulates actin assembly by stimulating the activity of the small GTPase, RacC. Yeast two-hybrid and biochemical experiments showed that active RacC binds to both Fbp17 and WASP. Inducing the expression of constitutively active RacC in cells produces effects similar to GxcM overexpression, in Fbp17- and WASP-dependent manner. Furthermore, deletion of racC causes phenotypes similar to deletion of fbp17, resulting in defects in cortical integrity and function.
In summary, this study delineates a signaling cascade composed of GxcM, Fbp17, RacC, WASP, and Arp2/3, which regulates the formation of the rear cortical subcompartment in rapidly migrating cells and maintains cortical integrity. Therefore, apart from its well-defined role in formation of the protrusions at the cell front, the Arp2/3 complex-based actin carries out a previously unappreciated function in building the rear cortex. How the same core actin polymerization machinery drives the formation of different types of cellular structures and, thus, mediates diverse cell functions remains an outstanding question for future studies.
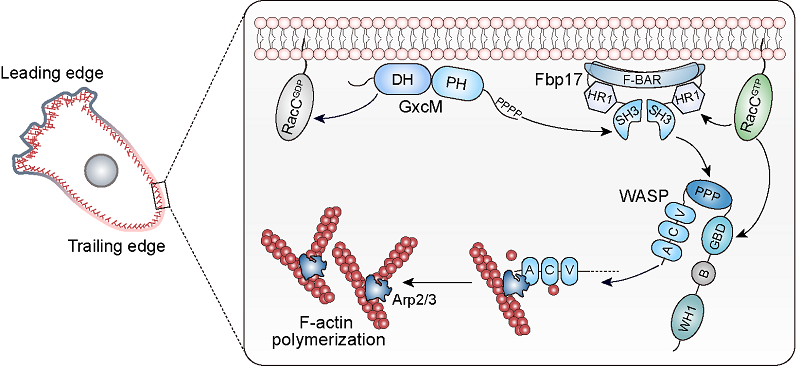
Fig 2. GxcM functions together with Fbp17, RacC, and WASP to coordinately promote Arp2/3 complex-mediated formation of the rear cortical subcompartment in rapidly migrating cells.
Full text link: https://doi.org/10.1083/jcb.202208151
Contact: CAI Huaqing
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: huaqingcai@ibp.ac.cn
(Reported by Dr. CAI Huaqing's group)

