Breakthrough Study Unveils Bacteriophages' Strategy to Counteract Hosts
In the never-ending arms race between bacteriophages and their prokaryotic hosts, prokaryotes have developed diverse defense systems to fend off phage invasions. These defense systems include the restriction-modification (R-M) system, CRISPR-Cas system, and newly identified systems causing abortive infection, among others. Notably, the CRISPR-Cas system represents the only adaptive immune system. Consequently, phages have evolved anti-CRISPR proteins to counteract this system.
The CRISPR-Cas systems can be classified into two classes and six types. The Cas9 effector protein from the type II system has gained widespread use in genome editing due to its high efficiency and simplicity. Numerous anti-CRISPR proteins that inhibit Cas9 have been identified and characterized. Understanding the mechanisms of these anti-CRISPR proteins will deepen our knowledge of the intricate relationship between phages and their hosts and facilitate the development of gene editing tools.
On July 26, a team led by Prof. WANG Yanli from the Institute of Biophysics, Chinese Academy of Sciences, reported significant progress in their research on an anti-CRISPR protein called AcrIIC4. This protein effectively inhibits type II-C Cas9 by preventing the formation of R-loops. The findings of this study were published online in PNAS.
The AcrIIC4 protein is encoded by prophages found in Haemophilus parainfluenzae (Hpa) and exhibits broad-spectrum inhibition capabilities. Both in vitro and in vivo experiments have confirmed AcrIIC4's inhibitory potency, indicating its potential as a regulatory tool for genome editing. However, due to the absence of a high-resolution structure of the Cas9-AcrIIC4 complex, the exact mechanism of AcrIIC4's inhibition remains elusive.
In this study, the researchers successfully solved the crystal structure of AcrIIC4 bound to the HpaCas9-sgRNA surveillance complex. AcrIIC4 occupies the crevice between the recognition domains (REC1 and REC2) and forms extensive interactions with sgRNA. This binding restricts the movement of the flexible REC2 domain.
To assess the effect of AcrIIC4 binding, the researchers also determined the structures of HpaCas9-sgRNA-DNA with and without AcrIIC4. Structure comparison revealed that the DNA:RNA heteroduplex could only partially form in the presence of AcrIIC4 but fully formed in its absence. AcrIIC4 effectively halts the propagation of the DNA:RNA heteroduplex, preventing the formation of an intact R-loop, which is crucial for the successive allosteric activation of Cas9.
Furthermore, the researchers conducted comprehensive biochemical assays to investigate AcrIIC4's effect on target DNA binding. The results demonstrated that AcrIIC4 prevents the unwinding of double-stranded (ds) DNA at the PAM-distal end and also affects the formation of the DNA:RNA binding channel. Although AcrIIC4 reduces the binding of DNA to Cas9, it does not abolish it, thus clarifying previous controversies regarding AcrIIC4's role in preventing DNA binding.
Intriguingly, AcrIIC4 exhibits varying degrees of inhibition on different type II-C Cas9 orthologs. The researchers discovered that distinct lengths of stem loop 1 account for this variation, a finding that holds significance for genome editing applications using various Cas9 orthologs when employing AcrIIC4 for regulation.
In conclusion, AcrIIC4 employs a novel mechanism to inhibit Cas9 cleavage. Unraveling its mode of action not only enhances our understanding of the ongoing phage-host interactions but also expands the repository of tools available for genome editing.
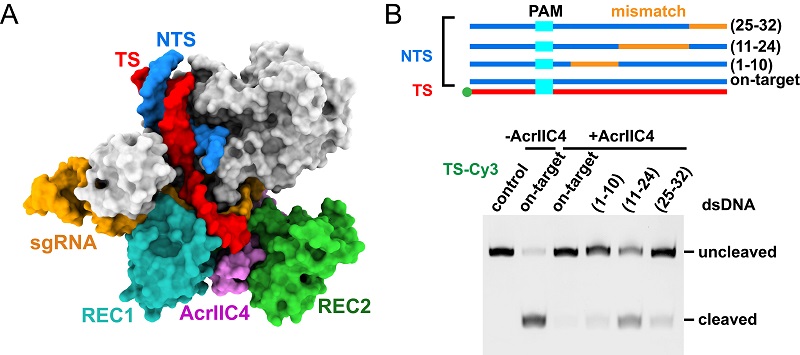
Figure. Structural and biochemical evidences illuminating the mechanism of action of AcrIIC4. (A) Cryogenic-electron microscopy structure of Cas9-sgRNA-DNA in the presence of AcrIIC4. (B) Cleavage assay showing that AcrIIC4 prevents dsDNA unwinding at the PAM-distal end.
Article link: https://www.pnas.org/doi/10.1073/pnas.2303675120
Contact: WANG Yanli
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: ylwang@ibp.ac.cn
(Report by Prof. WANG Yanli's group)

