The group of Prof. Sarah Perrett and collaborators have revealed the modulation mechanisms of methylene blue on the phase separation and aggregation of tau
Biomacromolecules such as proteins and nucleic acids form highly dynamic and concentrated liquid-like microcompartments in the cell via liquid-liquid phase separation (LLPS), which correlates with a wide range of biological functions. On the other hand, abnormal phase separation is implicated in the formation of the pathological aggregates related to neurodegenerative diseases. It has been reported that the proteins associated with degenerative diseases, including TDP-43, FUS, and Tau, undergo liquid-liquid phase separation in vivo and in vitro, promoting the liquid-to-solid conversion and the formation of pathological aggregates. Given the correlations between protein LLPS and pathological aggregation, development of the small molecules targeting the process of LLPS are expected to provide new clues for the prevention and treatment of neurodegenerative diseases.
The microtubule-associated protein tau is an intrinsically disordered protein that plays an essential role in microtubule assembly and stabilization. Under pathological conditions, tau protein forms amyloid aggregates. Recent studies have also shown that the LLPS of tau may be the triggering factor leading to the amyloid aggregation of tau. Inhibition of amyloid aggregation of tau or disaggregation of toxic aggregates by small molecules is one of the strategies for the treatment of tauopathies. It has been found that methylene blue (MB) and its derivative LMTM can effectively inhibit the amyloid aggregation of tau protein, but whether MB and its derivatives exert their amyloid inhibition activity via modulating the phase separation of tau is unclear. Given the complicated relationship between tau phase separation and its functional and pathological aspects, it is important to study the regulatory effects of MB and its derivatives on the LLPS of tau, in order to reveal the intermolecular interaction mechanisms involved in this process and its influence on the amyloid aggregation of tau. In this study, the group of Prof. Sarah Perrett and Prof. WU Si from the Institute of Biophysics, CAS collaborated with the group of Prof. HUANG Yongqi from Hubei University of Technology to systematically investigate the inhibition mechanism of tau aggregation via modulating LLPS of tau by MB. The researchers found that MB and its derivative LMTM can significantly promote the LLPS of tau. FRAP and optical tweezers experiments revealed that MB accelerates the gelation of tau droplets in the LLPS state, independent of the redox activity of MB. Further studies by NMR, FRET and molecular docking revealed that MB interacts extensively with multiple domains of tau, promoting the conformation extension of tau and the formation of intermolecular electrostatic and hydrophobic interaction networks. In contrast to the previous observations on other amyloid proteins, MB-induced LLPS and gelation of tau delays the formation of toxic amyloid fibrils and reduces the cytotoxicity without any effect on the function of tau in promoting microtubule assembly. These findings suggest that MB inhibits tau amyloid fibrillization and accelerates tau droplet gelation via distinct mechanisms, thus providing insights into the activity of tau aggregation inhibitors in the context of phase transition.
This study titled "Methylene blue accelerates liquid-to-gel transition of tau condensates impacting tau function and pathology" was published on-line in the journal Nature Communications on 6 September 2023. Dr. WU Si from the Institute of Biophysics, CAS, Dr. HUANG Yongqi and Dr. GAO Meng from Hubei University of Technology are the corresponding authors. Dr. HUANG Yongqi and Postdoctoral Fellow WEN Jitao from the group of Prof. Sarah Perrett are the co-first authors. The group of Prof. Markus Zweckstetter from Max Planck Institute, Germany, the group of Prof. Hanbin Mao from Kent State University and Prof. Junmei Wang from University of Pittsburgh, USA made contributions to the NMR, optical tweezers experiments and the molecular docking in this work. The work was funded by the National Natural Science Foundation of China and the Ministry of Science and Technology of China.
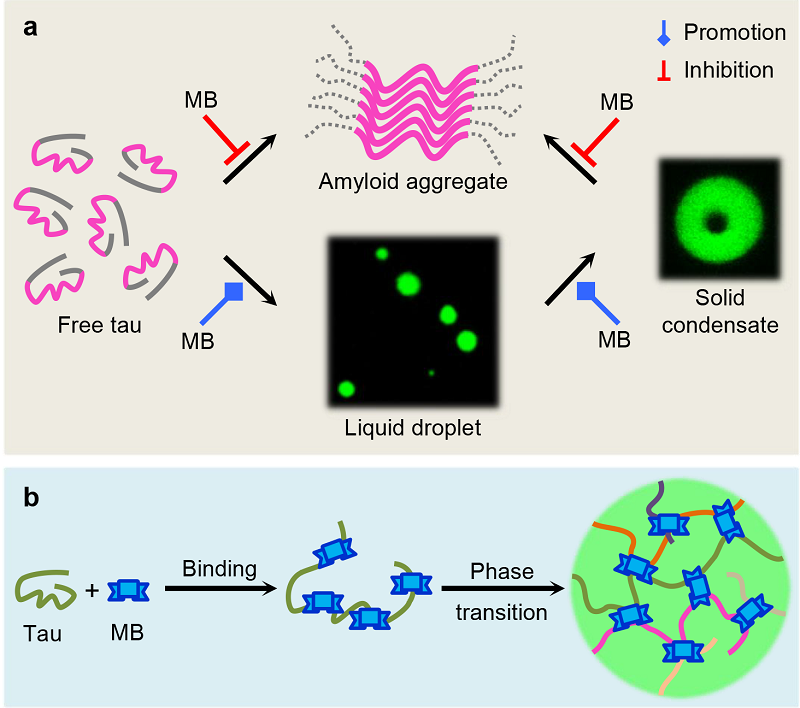
Figure. Illustration of MB-regulated tau phase separation
Article link: https://www.nature.com/articles/s41467-023-41241-6
CONTACT:
Si Wu, Sarah Perrett
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64888496
Email: wusi@ibp.ac.cn; sperrett@ibp.ac.cn
(Reported by Prof. Sarah Perrett's group)

