Researchers revealed the controlled self-assembly mechanism of natural silk fiborin in silkworm programmed by metal ions and pH gradient
Silk-secreting animals such as silkworms and spiders possess similar spinning organs and spinning mechanisms. They use water-soluble silk proteins as raw materials to produce fibers with excellent strength and toughness at room temperature and pressure through specific assembly and structural transformation. For many years, researchers have been working on revealing the spinning mechanism of silkworms and spiders, in the hope of mimicking nature's solutions to synthesize artificial fiber materials that can match or even surpass the mechanical properties of natural silk.
Currently, researchers have proposed two types of spinning model, namely "liquid crystalline spinning" (Vollrath, et al., Nature, 2001) and "micelle" spinning (Jin, et al., Nature, 2003). The two spinning models differ in the ultra structure and assembly process of silk protein prior to fibrillation. Due to the difficulty of accurately observing the ultra structure of natural silk fibroin (NSF) in the lumen of silk glands, it is highly controversial on the fundamental building blocks making up the spinning dope, and how these building blocks assemble to form silk fiber.
The most difficult aspect of studying the morphology and assembly of NSF of silkworm is that NSF is able to remain stable at high concentration of 15%-30% (w/v) without precipitation in vivo, whereas it readily transforms from random coil to β-sheet structure in vitro, resulting in protein precipitation, and ultimately making it difficult to study the structure and assembly mechanism of NSF.
On December 29, 2023, a research paper entitled "Decoding silkworm spinning programmed by pH and metal ions" was published on 《Science Bulletin》by a team lead by Prof. ZHU Ping at the Institute of Biophysics, Chinese Academy of Sciences, and Prof. XIA Qingyou and Prof. HE Huawei at Southwest University in China. Through large-scale screening, the researchers managed a way to keep the structure of NSF stabilized in vitro. On this basis, a systematic and detailed study was conducted on the morphology and structure of NSF and their associated modulating factors in the silkworm. The researchers revealed that the spinning dope of silkworm is built by flexible nanofibrils with a diameter of about 4 nm, other than the micellar or rod-like structures in the previously proposed models. Induced by metal ions, NSF polypeptide chains reversibly fold into nanofibrils predominantly composed of random coils. The successive pH decrease from the posterior of silk gland (PSG) to the anterior of silk gland (ASG) resulted in a gradual increase in NSF hydrophobicity, thus inducing the sol-gelation transition of NSF nanofibrils, which eventually contributes to the increase of NSF concentration and fiber formation. More importantly, the researchers found that NSF nanofibrils were randomly dispersed from PSG to ASG-1, but self-assembled into anisotropic herringbone patterns at ASG-2 near the spinneret, in which the adjacent NSF nanofibrils were aligned parallel to each other (Fig. 1), which is finally prepared for the silk spinning.
Taken together, these findings reveal a self-assembly mechanism of the multi-scale hierarchical architecture of NSF in silkworm programmed by metal ions and pH gradient, which delivers novel insights into silk-secreting animals spinning and bionic design of high-performance fibers.
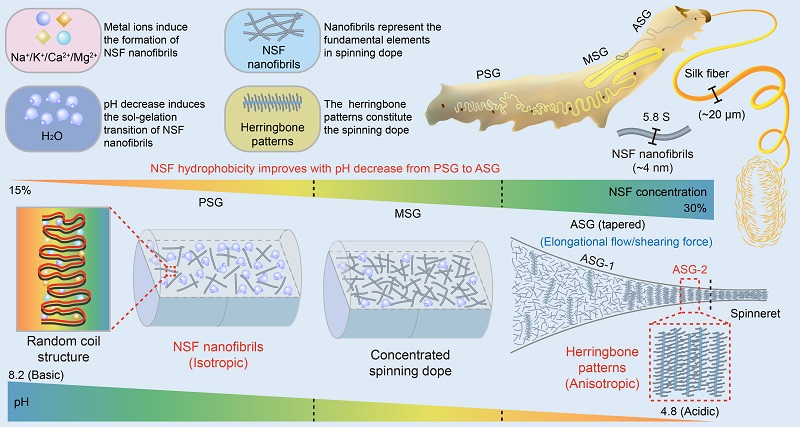
Fig. 1: A schematic diagram of silkworm spinning
Dr. SONG Kai (currently a postdoctoral fellow in the group of Prof. ZHU Ping), jointly trained by the Southwest University and the Institute of Biophysics, is the first author. Prof. ZHU Ping at the Institute of Biophysics, and Prof. XIA Qingyou and Prof. HE Huawei at the Southwest University are the co-corresponding authors of the paper. This work was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China and the Chinese Academy of Sciences (CAS) Strategic Priority Research Program.
Article link: https://doi.org/10.1016/j.scib.2023.12.050
Contact: Ping Zhu
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: zhup@ibp.ac.cn
(Reported by Prof. ZHU Ping's group)

